Patients with mechanical heart valves (MHVs) require chronic anticoagulation to reduce the risk of ischemic stroke and systemic thromboembolism. Warfarin is the only oral therapeutic option in this population, with poor outcomes associated with trials of dabigatranReference Eikelboom, Connolly and Brueckmann1 and dual antiplatelet therapy.Reference Puskas, Gerdisch and Nichols2 Based on the valve location, the majority of North American patients with MHVs are targeted to an international normalized ratio (INR) range of either 2.0–3.0 or 2.5–3.5. The most feared complication of warfarin therapy is intracranial hemorrhage. Patients with MHVs who develop an intracranial hemorrhage while therapeutically anticoagulated require INR reversal to prevent further hematoma expansion. This poses a complicated clinical dilemma as clinicians must balance the risk of continued or rebleeding with the high thromboembolic risk associated with MHVs. At present, published data on post-intracranial hemorrhage antithrombotic management have focused on timing and strategies for INR reversal and the optimal time to reinitiate therapy.Reference Kuramatsu, Sembill and Gerner3,Reference Chandra, Gupta and Grover4 Guidance on INR targets post-intracranial hemorrhage is limited.
The Anticoagulation Management Service (AMS) in Alberta, Canada has provided antithrombotic management to over 4400 patients since its inception in 2001. From 2003 onwards, the AMS received direct referrals for all MHVs implanted in Edmonton and currently manages over 600 patients with MHVs. We describe a case series of warfarin-associated intracranial hemorrhages, clinical outcomes, and post-intracranial hemorrhage antithrombotic therapy decisions.
We conducted a retrospective review of the AMS database. Eligible patients were taking warfarin at the time of intracranial hemorrhage, had antithrombotic treatment managed by the AMS for at least three-month post-hemorrhage, and were 18 years or greater. Medical records were reviewed from both the AMS and the hospitals where the patients presented. The protocol was approved by the Health Research Ethics Board at the University of Alberta (Pro00089835).
We identified 13 cases between April 2005 and October 2018. Four patients had a mechanical aortic valve, six patients had a mechanical mitral valve, and three patients had both valves (Table 1). All known valve types were bileaflet (N = 9). The median age was 59 years (interquartile range (IQR): 15 years), many had atrial fibrillation/flutter (AF, N = 8), half had a history of hypertension (N = 7), few had chronic kidney disease (N = 3), and none had hepatic disease. Prior to presentation with intracranial hemorrhage, the target INR range was 2.0–3.0 for 3 patients (2 aortic, 1 mitral MHV), 2.5–3.5 for 10 patients (1 aortic, 6 mitral, 3 with both MHVs), and concomitant acetyl salicylic acid (ASA) administered in 8 patients (3 aortic, 4 mitral, 1 with both MHVs; none had a history of coronary artery disease, previous acute coronary syndrome or myocardial infarction). The median time to presentation from MHV implant to intracranial hemorrhage presentation was 9 years, 3.5 months (IQR: 12 years, 5 months).
Table 1: Anticoagulant-associated intracranial hemorrhage case series results
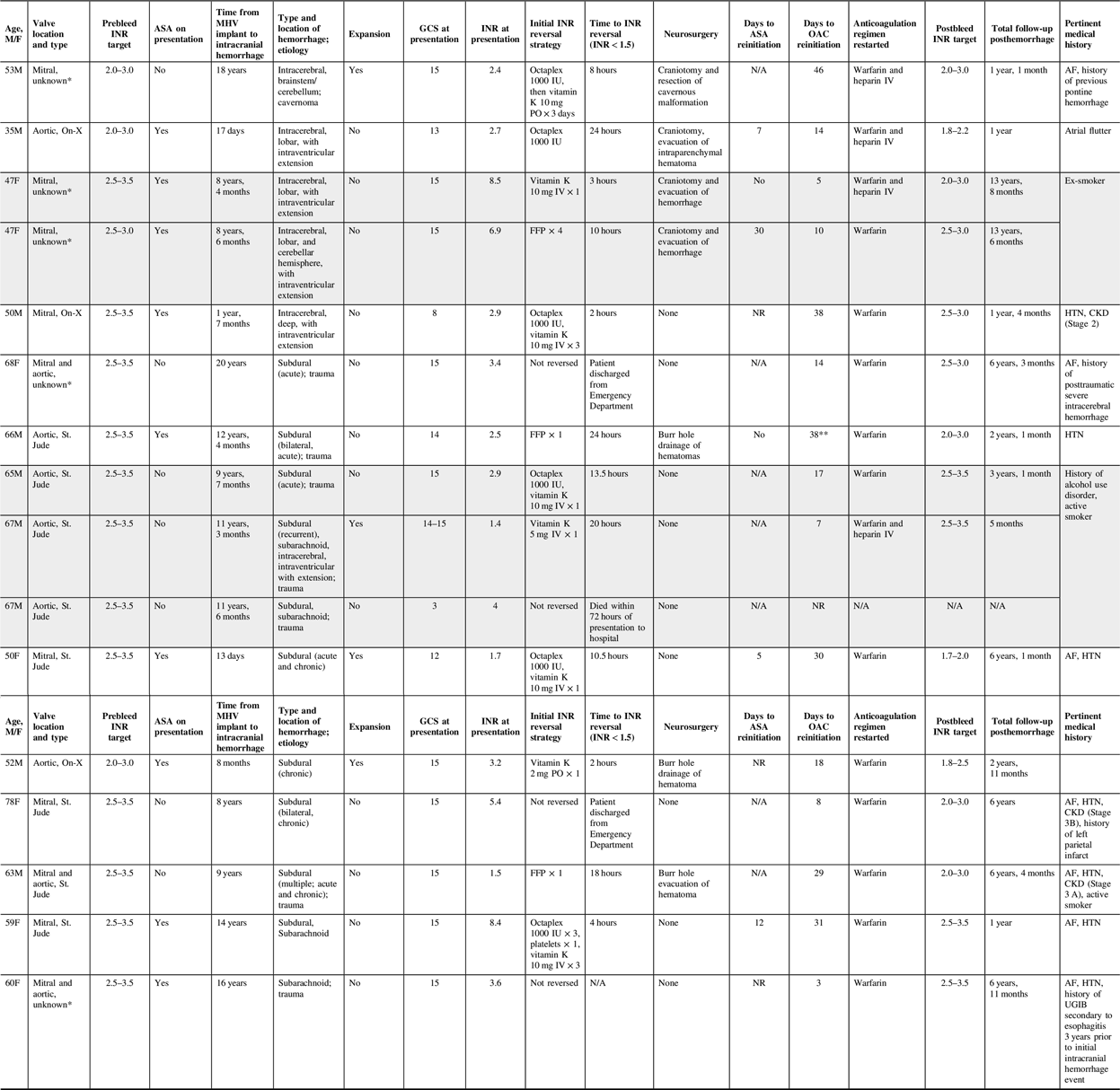
Shaded rows indicate patients who had recurrent intracranial hemorrhage.
AF = atrial fibrillation; ASA = acetyl salicylic acid; CKD = chronic kidney disease; FFP = fresh frozen plasma; GCS = Glasgow Coma Scale; HTN = hypertension; INR = international normalized ratio; IV = intravenous; MHV = mechanical heart valve; NR = not restarted; OAC = oral anticoagulant; Octaplex = a manufactured four-factor prothrombin complex concentrate derived from human plasma; PO = oral; UGIB = upper gastrointestinal bleed.
* Valve type not documented in charts reviewed.
** Patient was initiated on low molecular weight heparin (LMWH) for venous thromboembolism (VTE) prophylaxis 3 days after event and continued until OAC initiation.
The median Glasgow Coma Scale (GCS) score at the time of presentation with intracranial hemorrhage was 15 (IQR: 1.25). Of the 13 incident cases, 4 were intracerebral hemorrhages, 8 were subdural hemorrhages (4 were traumatic, 1 had subarachnoid bleeding), and 1 was a traumatic subarachnoid hemorrhage. The initial INRs varied, ranging from 1.4 to 8.4. Based on the target INRs prior to the inciting event, three INRs were subtherapeutic, seven were within range, and six were supratherapeutic upon presentation to a hospital. Anticoagulant reversal was attempted in 12 patients, and the INR was successfully reversed in less than 4 hours (N = 3) and greater than 4 hours (N = 9) irrespective of the reversal strategy used (e.g., prothrombin complex concentrate (PCC), vitamin K, fresh frozen plasma (FFP)). There were three recurrent hemorrhages in two patients. The median time to recurrent intracranial hemorrhage events was 3 months (IQR: 9 months). Following the initial computerized tomography (CT) scan, surgical procedures were completed without further complications (N = 7).
All patients who survived the index event were reinitiated on therapeutic anticoagulation with either warfarin and intravenous heparin (N = 4) or warfarin alone (N = 11) (median time to reinitiation: 17 days, IQR: 21.5 days). In trauma-related and intracerebral hemorrhages, the median time to reinitiation was 29 (IQR: 17 days) and 14 days (IQR: 28 days), respectively. Only one patient received pharmacologic venous thromboembolism prophylaxis before therapeutic anticoagulation.
Post-intracranial hemorrhage, the INR target range was modified in nine patients (INR target lowered (N = 4, one aortic, two mitral, one with both MHVs), lowered and narrowed (N = 3, two aortic, one mitral MHV), or narrowed (N = 2, one mitral, one with both MHVs)). 75% and 56% of patients with the same or modified INR post-intracranial hemorrhage had AF, respectively. Of the eight patients on concomitant ASA and warfarin at the time of presentation with intracranial hemorrhage, four (one aortic, three mitral MHVs) were restarted post-intracranial hemorrhage (median time: 9.5 days, IQR: 10 days). Upon discharge, the median total follow-up by the AMS post-intracranial hemorrhage was 3 years 1 month (IQR: 5 years, 1 month).
Overall, this case series of 13 patients with MHVs who developed anticoagulant-associated intracranial hemorrhage was heterogeneous in terms of type of intracranial hemorrhage, INR reversal strategy employed, timing of warfarin reinitiation, post-intracranial hemorrhage INR target, and concomitant ASA therapy. There were no obvious practice patterns with respect to MHV location, type and location of intracranial hemorrhage, hematoma expansion, or AF history and the timing of anticoagulant reinitiation, post-intracranial hemorrhage INR targets, or ASA reinitiation. Despite the heterogeneity, the majority of patients presenting with a GCS of 15 recovered without subsequent hemorrhagic or thromboembolic complications.
The majority of our cases presented with either therapeutic (N = 7) or supratherapeutic (N = 6) INRs, consistent with the literature reporting that a majority (50–90%) of warfarin-associated intracranial hemorrhage occurs within the target range.Reference Yung, Kapral and Asllani5 Within our cases, reversal strategies were variable and generally ineffective in terms of rapid INR reversal; only three patients achieved INR reversal within 4 hours of diagnosis. This may reflect changes in practice patterns over the course of this case series. Over time, PCC has replaced FFP and vitamin K alone as the recommended therapy in patients with warfarin-associated intracranial hemorrhage. However, even PCC appeared to take time to reverse INR in our case series. This is also consistent with published data where INR reversal with PCC can take as long as 1610 minutes.Reference Steiner, Poli and Griebe6
In our case series, the anticoagulation regimen (warfarin monotherapy, warfarin with intravenous heparin bridging (with and without bolus)), and time to restart was widespread (range: 3–46 days). There are no randomized studies of reinitiation of anticoagulation after intracranial hemorrhage in patients with MHVs, and observational data are fragmented. Specifically, Chandra et al. reported that therapeutic heparin can be restarted 3 days following intracranial hemorrhage and switched to warfarin at day 7 without concerns for major bleeding,Reference Chandra, Gupta and Grover4 while a more recent study by Kuramatsu et al. reported an increased incidence in hemorrhagic complications when therapeutic anticoagulation was restarted in less than two weeks.Reference Kuramatsu, Sembill and Gerner3 Patients in the case series by Babikian et al. resumed warfarin after a mean interval of 19 days, and thromboembolic events were not observed.Reference Babikian, Kase and Pessin7 Irrespective of the time to therapeutic anticoagulation restart, the outcomes of patients in our case series following intracranial hemorrhage were positive. Patients neither experienced a thromboembolic complication, nor an acute rebleed. The recurrent bleeding rate was low (N = 2) and occurred months following reanticoagulation (median: 3 months, IQR: 9 months). These findings were similar to a study by Phan et al. where patients at high embolic risk had a low probability of embolic events when warfarin therapy was discontinued for one to two weeks and intracranial hemorrhage recurrence was uncommon.Reference Phan, Koh and Wijdicks8 Our study suggests that the likelihood of both early recurrent hemorrhage and thromboembolic events is lower than previously suspected (the thrombogenicity of more modern MHVs may be less than their historical counterparts). It is therefore reasonable to reverse the therapeutic coagulopathy in cases of intracranial bleeding and restart once considered stable by the management team.
Unlike previous case series, we assessed INR target modifications post-intracranial hemorrhage (N = 9). The majority of our patients had INR target ranges lowered (N = 4), lowered and narrowed (N = 3), or narrowed (N = 2). A lower INR target has been suggested after anticoagulant-associated intracranial hemorrhage, although this is largely based on opinion rather than evidence.Reference Li and Lip9 One other case series indicated that 69% of patients had a lower INR target post-intracranial hemorrhage (pre-intracranial hemorrhage INR target and subsequent correlation with patient outcomes were not documented).Reference Butler and Tait10 Our case series reports that both patients with the same or modified INR target post-intracranial hemorrhage did not result in any subsequent thromboembolic events. Similarly, patients reinitiated on concomitant ASA and warfarin therapy or reduced to warfarin monotherapy did not experience any subsequent rebleeding or thromboembolic event in follow-up. While narrowing the target range may be more achievable in an AMS, this approach likely mandates more frequent INR testing and mitigates larger fluctuations in sub/supratherapeutic INRs when compared to a wider target range.
Limitations of this case series are its small sample size and retrospective design. Data collection based on chart review limits the precision of the results presented (e.g., variability in number and timing of follow-up INR testing and CT scans).
In conclusion, antithrombotic management following intracranial hemorrhage among our cases was variable, and neither correlated with the type, location, or etiology of bleed, nor the valve type and associated thromboembolic risk. Diligent assessment and management of INR may improve long-term outcomes more than timing of warfarin restart and/or modified target INR.
Conflict of Interest
AW, MH, and KS have nothing to disclose.
KB has received speaker honoraria, served on advisory boards, and received research grant support from Boehringer-Ingelheim, Bayer, Pfizer, and Servier.
TJB has received speaker honoraria from Bayer and unrestricted research grants from Bayer and Pfizer.
Statement of Authorship
AW contributed to the concept and design, analysis, or interpretation of the data, critical revision with intellectual content, and final approval of the version to be published.
KB contributed to the design, analysis, or interpretation of the data, critical revision with intellectual content, and final approval of the version to be published.
MH contributed to the interpretation of the data, critical revision with intellectual content, and final approval of the version to be published.
KS contributed to the concept, interpretation of the data, critical revision with intellectual content, and final approval of the version to be published.
TJB contributed to the concept and design, analysis, or interpretation of the data, critical revision with intellectual content, and final approval of the version to be published.



