Stroke is a leading cause of mortality and disability worldwide. Projections indicate that the total number of stroke events will increase by more than 30% in the next years.Reference Owens Johnson, Nguyen and Roth1
Over the past 4 years, endovascular thrombectomy (EVT) trials reported large treatment benefits in selected patients. However, the effect of EVT performed later than 6 hours after the onset of symptoms was uncertain. Recent trials shed light on this aspectReference Nogueira, Jadhav and Haussen2,Reference Albers, Marks and Kemp3 : endovascular treatment plus standard medical therapy results in better functional outcomes also in patients with acute stroke due to large vessel occlusion (LVO), last known well 16 hoursReference Albers, Marks and Kemp3 or even 24 hoursReference Nogueira, Jadhav and Haussen2 earlier. Actually, the efficacy of these treatments is limited only to patients carefully selected with neuroimaging techniques. Therefore, EVT is now performed in witnessed, unwitnessed, or wake up strokes who have a mismatch between clinical deficit and infarct or between the ischemic core and penumbral regions.
Herein, we describe two cases of wake-up stroke in a 39-year-old woman and in a 17-year-old girl, both with mild symptoms but relevant neuroradiological mismatch.
A 39-year-old woman with Eisenmenger’s syndrome, caused by patent ductus arteriosus treated surgically during her childhood, and frequent abnormal vaginal bleedings was admitted to our hospital with dysarthria and left-sided hemiparesis on awakening (NIHSS 5). An episode of uncertain coffee-ground vomitus occurred that morning. Cerebral MRI and angio-MRI were performed, suggesting recent cerebral infarction due to distal M1 segment occlusion of the right middle cerebral artery (MCA); the mismatch was favorable (Figure 1C,D). Right fronto-opercular and lenticular ischemic lesions were detected on diffusion-weighted imaging (DWI) (Figure 1A), with only hyperintense vessel or “Spaghetti sign” – an early sign of acute ischemic stroke – on fluid-attenuated inversion recovery (FLAIR, Figure 1B).
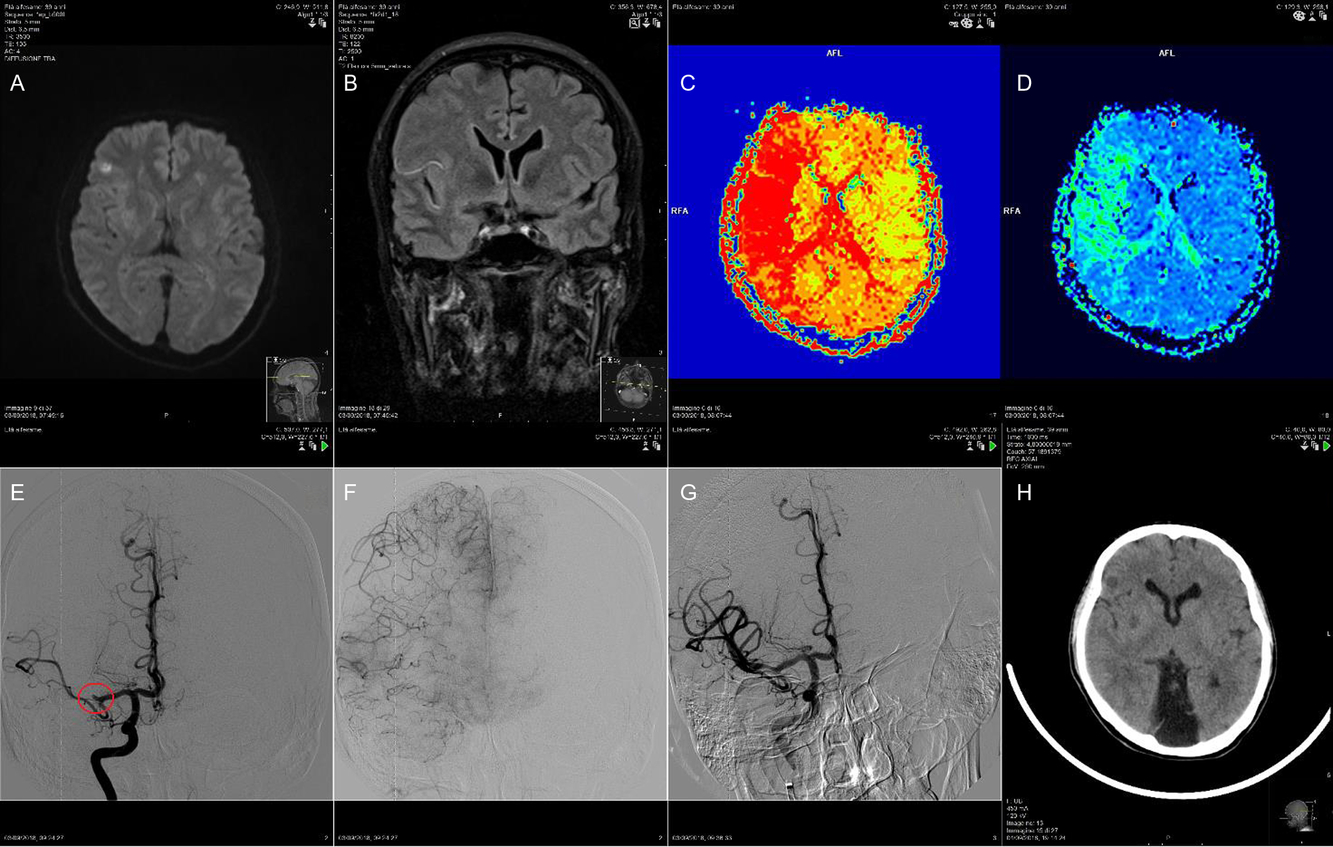
Figure 1: Right fronto-opercular and lenticular ischemic lesions were detected on DWI (A); hyperintense vessel or “Spaghetti sign” on FLAIR (B); MRI perfusion-diffusion (PWI-DWI) showed a favorable mismatch (C and D); cerebral angiography suggested the distal M1 segment occlusion of the right MCA (E); angiographic evaluation of collaterals in the right hemisphere of the brain (F); TICI IIb final angiographic recanalization after the mechanical thrombectomy (G); cerebral CT scan carried out the next day showed only the ischemic lesions already seen on MRI DWI (H).
Intravenous alteplase was not administered because of the episode of coffee-ground vomitus and the history of frequent abnormal vaginal bleedings. Thus, a mechanical thrombectomy using aspiration only was performed, resulting in a thrombolysis in cerebral infarction (TICI) IIb final angiographic recanalization. A cerebral CT scan carried out the next day showed only the ischemic lesions already seen on MRI DWI. The patient recovered completely.
The second case is a late teenage patient, with limb-girdle muscular dystrophy (LGMD) associated with hypertrophic/dilated cardiomyopathy that had required an implantable cardioverter defibrillator (ICD). She was admitted to our hospital for right hemiparesis and aphasia (NIHSS 9) on awakening. Because of her ICD, cerebral MRI could not be performed. An emergency CT angiography revealed occlusion of the left M1 segment of MCA (Figure 2A); a hypoperfused region of the brain with favorable mismatch were detected on perfusion imaging in the territory of the left MCA (Figure 2C,D). Thrombectomy was performed using a stent retriever. After a single pass, the clot was completely removed and the flow in the M1 was completely restored, resulting in a TICI III (Figure 2B). The next day, cerebral CT scan showed an infarction in the left lenticular nucleus and in the posterior corona radiata (Figure 2E). Cardiac monitoring identified atrial fibrillation (AF). The patient was discharged with final NIHSS 0 and mRS returned to 1 such as her pre-stroke mRS related to LGMD.
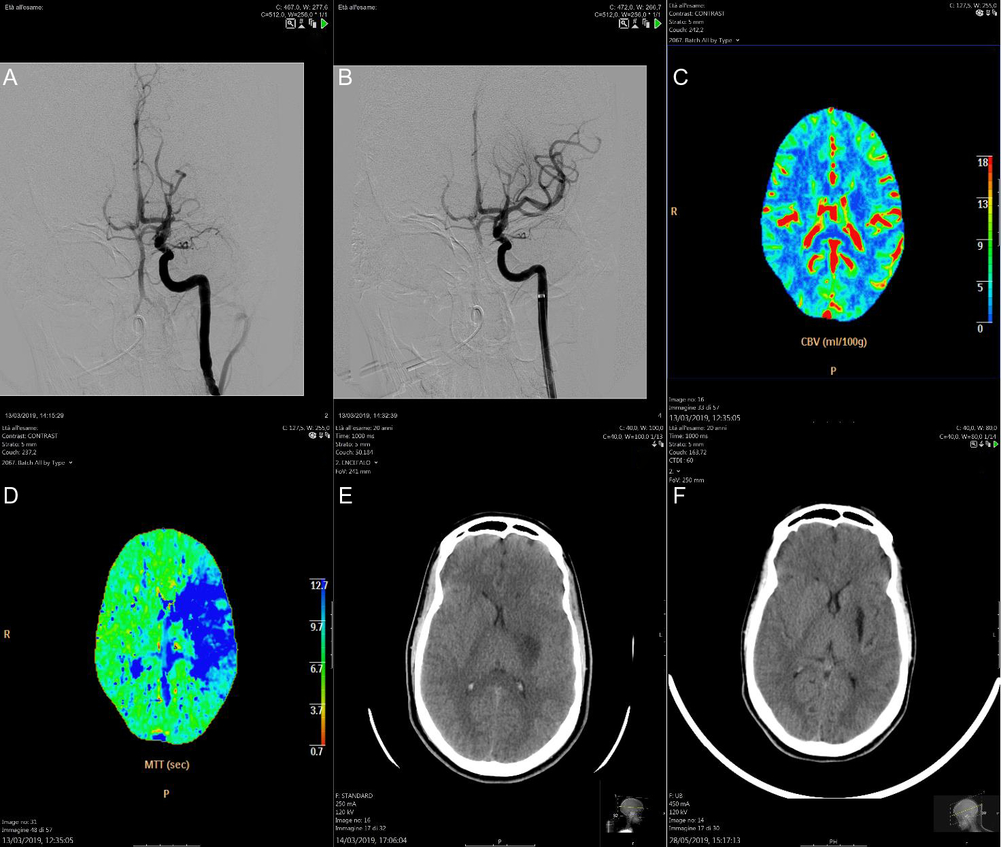
Figure 2: Cerebral angiography revealed occlusion of the left M1 segment of MCA (A); after the thrombectomy, the clot was completely removed and the flow in the M1 was completely restored, resulting in a TICI III (B); cerebral perfusion CT detected a hypoperfused region of the brain with favorable mismatch in the territory of the left MCA (C and D); the next day, cerebral CT scan showed an infarction in the left lenticular nucleus and in the posterior corona radiata (E); after 2 months, the ischaemic lesion became more evident and hypodense on the cerebral CT scan (F).
Over the last years, guidelines on acute stroke treatment have been quickly modified. It is now well known that EVT is effective among patients with a LVO and NIHSS ≥ 6. In the EVT group of DEFUSE 3 and DAWN trial, median NIHSS score was 16 and 17, respectively.Reference Nogueira, Jadhav and Haussen2,Reference Albers, Marks and Kemp3 Conversely, EVT benefit for LVOs with mild deficits (NIHSS < 6) is still uncertain and the American Heart Association/American Stroke Association (AHA/ASA) guidelines recommend this treatment only in patients with NIHSS ≥ 6.Reference Sarraj, Hassan and Savitz4 However, the recent European Stroke Organization (ESO) guidelines do not anymore recommend a NIHSS score limit for decision-making on EVT and suggest – as an expert opinion – that treatment with mechanical thrombectomy may be reasonable in patients with deficits that appear disabling or in the case of clinical worsening despite intravenous thrombolysis.Reference Turc, Bhogal and Fischer5 Moreover, differences between national guidelines on this matter still exist.
Thus, both our patients could be treated in different ways depending on the guideline that the clinician decided to follow.
However, they could not be candidate for any treatment before these recent studies.Reference Nogueira, Jadhav and Haussen2,Reference Albers, Marks and Kemp3,Reference Thomalla, Simonsen and Boutitie6 In fact, our first patient was admitted with only mild symptoms on awakening: there were no strong recommendations in these cases yet, but the decision to treat or not a patient should be discussed together with stroke experts, considering all the features of every clinical case.
Regarding stroke in children and adolescents, currently there are no clear recommendations, despite overwhelming data suggesting that EVT performed in the adult population is safe.Reference Bhogal, Hellstern and AlMatter7 Recruiting pediatric patients to such a trial is difficult, so the efficacy of EVT is yet to be proven in the pediatric cohort.
However, in our experience, EVT should be an option in this population, especially in older children with typical pathophysiological mechanism: in our case, we assumed that the stroke was probably cardioembolic due to her underlying myopathy. In fact, the prevalence of AF in patients with primary myopathies is about 15%,Reference Chen, Tang, Su, Yang and Jeng8 and during hospitalization AF was detected and a proper anticoagulation was started. Indeed, the operative technique required is usually the same as in adult patients, also considering the enormous benefit they could receive if successfully treated in terms of quality of life.
Hence, these clinical scenarios remain controversial. For this reason, to highlight potential advantages and disadvantages of EVT in these particular groups, individual case reports are very important.
In conclusion, in our opinion, clinical reports of challenging patients treated with EVT and hard to recruit in a trial, provide precious information, play an important role in improving health outcomes and can guide the clinicians in difficult choices during the routine clinical practice. This underscores the relevance of data collection in dedicated registries that better delineate the application in “real-life” patients of interventions found to be effective in very selected populations.
Disclosures
The authors have no conflicts of interest to declare.
Statement of Authorship
NR: Drafting of manuscript. Manuscript conception. Clinical investigator. Analysis and interpretation of data. MGF: Clinical investigator. Manuscript conception. Acquisition of data. Critical revision of the manuscript. DS: Clinical investigator. Served as scientific advisor and critically reviewed the manuscript. PB: Clinical investigator. Served as scientific advisor and critically reviewed the manuscript. VM: Clinical investigator. Served as scientific advisor and critically reviewed the manuscript. CS: Clinical investigator. Served as scientific advisor and critically reviewed the manuscript. AS: Department Chair. Manuscript conception. Analysis and interpretation of data. Served as scientific advisor. Critically reviewed the manuscript.




