Aneurysmal compression is the most feared etiology of third nerve palsy (TNP). We describe the first case of compressive TNP secondary to dolichoectactic posterior communicating artery (PCommA). Dolichoectasia refers to the elongation and tortuosity of a blood vessel with dilatation as its main pathological feature. We discuss the spectrum of pathological blood vessel dilation ranging from the saccular aneurysm to fusiform aneurysm and dolichoectatic vessels, and highlight dolichoectasia as a potential source for compressive TNP.
A 68-year-old man was presented to a neuro-ophthalmology clinic complaining of binocular diplopia. He also described noticing his left eye “wandering out” for the past 5 years. There was no known history of childhood strabismus or amblyopia and he denied a history of preceding trauma. On examination, the vision was 20/30 and 20/25. Pupils were equal in size and reactive to light with no relative afferent pupillary defect. There was mild ptosis of the left upper lid. On motility testing, supraduction was 80% normal in the left eye but otherwise, no abnormalities were observed. On alignment testing, there were 12 prism diopters (PD) of right hypertropia, which decreased in down gaze, along with 10 PD of comitant exotropia.
A diagnosis of partial pupil-sparing left TNP was suspected and an MRI and MR angiography of the brain and orbits with contrast was performed. It revealed bilateral, left more greater than right, dolichoectatic PCommA with the left PCommA abutting and displacing the third nerve (Figure 1). There was no aneurysmal dilatation of the affected vessel wall.
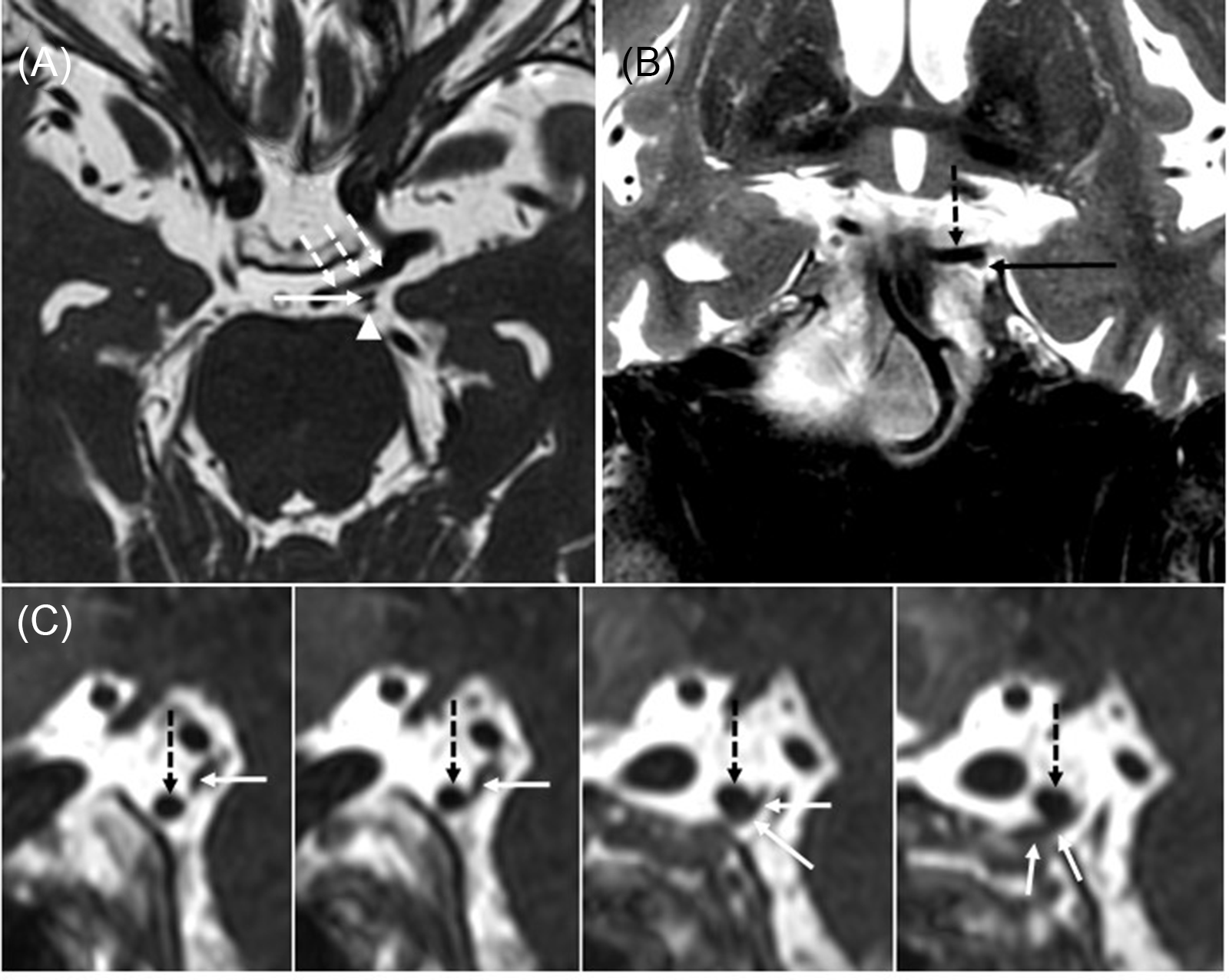
Figure 1: Large left posterior communicating (PComm) artery displacing the left oculomotor nerve. (A) Axial 3D FIESTA sequence shows a large left PComm artery (dashed arrows), the left oculomotor nerve (arrow), and the left superior cerebellar artery (arrowhead). (B) Coronal T2-weighted image shows the left oculomotor nerve (arrow) coursing along the undersurface of the left PComm artery (dotted arrow). (C) Series of sagittal reformats from the 3D FIESTA sequence shows the left PComm artery (dotted arrows) indenting the left oculomotor nerve (arrows).
The third cranial nerve exits the ventral surface of the midbrain between the posterior cerebral artery (PCA) and superior cerebellar arteries and runs just underneath the PCommA in the subarachnoid space. PCommA infundibulum, a funnel-shaped dilatation occurring at its site of origin from the ICA, is relatively common and has been described as a rare cause of compressive TNP. Reference Fukami, Akimoto, Fukuhara and Kohno1 This is considered as a normal anatomic variant with no risk of rupture. Aneurysmal dilation of the PcommA, most commonly occurring at the junction of Pcomm and internal carotid artery (ICA), is a well known and most feared cause of TNP, accounting for approximately 6% of acquired TNP cases. Reference Fang, Leavitt, Hodge, Holmes, Mohney and Chen2 Thus, all cases of TNP should have urgent CT or MR angiography performed in order to rule out PComm aneurysm, which carries a high risk of rupture after it becomes symptomatic. Aneurysms greater than 3-4 mm can be reliably detected using modern noninvasive imaging modalities, though conventional catheter angiography may be required if the diagnosis is uncertain or to better characterize the anatomy for choosing a treatment strategy.
In this case, the absence of pupillary involvement occurred in the presence of compressive etiology of TNP, emphasizing the fact that normal pupillary function in patients with TNP is neither sensitive nor specific for ruling out up compressive lesion, conforming to the literature describing the absence of pupillary involvement in up to one-third of patients with compressive lesions causing TNP. Reference Fang, Leavitt, Hodge, Holmes, Mohney and Chen2
Most intracranial aneurysms are saccular with a neck and focal bulging at one side of a vessel wall. The risk of saccular aneurysms rupture is significant due to the high flow within the outpouching and thinning of the involved vessel wall. Fusiform aneurysms occur when there is spindle-shaped dilation of a vessel with no neck thus the risk of their rupture is substantially lower. Apart from saccular and fusiform aneurysms, pathological elongation and tortuosity of a vessel without focal irregularities or aneurysmal outpouching can occur and is referred to as dolichoectasia. Reference Pico, Labreuche and Amarenco3
Dolichoectasia is usually considered to be within the spectrum of vessel abnormalities along with fusiform aneurysms. It is defined as an increase in the length and diameter of an intracranial artery. An exact cutoff for vessel diameter to be considered as dolichoectatic has been proposed for the basilar artery but has not been defined for the ICA and its branches. Reference Pico, Labreuche and Amarenco3 The prevalence varies from 0.05% to 6% with over 80% of intracranial dolichoectasia involving the vertebrobasilar system. Reference Pico, Labreuche and Amarenco3 Pathophysiology of dolichoectasia is unknown, but is considered to be the result of vessel wall’s response to various vascular risk factors and congenital vessel variations with changes in the tunica media due to matrix metalloproteinase activation and resultant injury of the muscle cells and elastic fibers. Dolichoectasia has been associated with older age, hypertension, coronary artery disease, and abdominal aortic aneurysms. Reference Pico, Labreuche and Amarenco3,Reference Kobkitsuksakul, Somboonnitiphol, Apirakkan, Lueangapapong and Chanthanaphak4
Dolichoectasia of the ICA and its branches is much less common and less well characterized than that affecting vertebrobasilar circulation. Dolichoectasia of the PCommA in its distal portion, where it connects with the ICA is exceedingly rare and has never been reported to cause TNP. Dolichoectasia of the proximal portion of PCommA, P1 segment of PCA, and distal ICA has been reported as a rare congenital anomaly that can lead to aneurysm formation later in life, Reference Nasr, Flemming, Lanzino, Cloft, Kallmes and Murad5 though none of these anomalies were present in our patient.
One recent meta-analysis reported a rupture risk of 0% in dolichoectasia of the vertebrobasilar arteries, compared with 3% for fusiform aneurysms. Reference Nasr, Flemming, Lanzino, Cloft, Kallmes and Murad5 However, while the rupture of dolichoectatic vessels almost never occurs, the prevalence of dolichoestasia in patients with ischemic stroke is much higher than in the general population, likely due to the abnormal flow within the elongated and dilated vessel, predisposing it to thrombus formation and atheromatous changes. Reference Pico, Labreuche and Amarenco3,Reference Nasr, Flemming, Lanzino, Cloft, Kallmes and Murad5,Reference del Brutto, Ortiz and Biller6 Dolichoectatic vessels can also rarely cause obstructive hydrocephalus and compressive cranial nerve palsies.
To our knowledge, this is the first published report of TNP due to compression by a dilated PCommA without evidence of an actual aneurysm. It illustrates that while PCommA aneurysm is critical to exclude as a cause of TNP, the so-called “neurovascular conflict” can occur in the absence of aneurysmal vessel dilation and the relationship between the third nerve and the surrounding vessels should be closely examined on neuroimaging.
Disclosures
The authors have no conflicts of interest to disclose.
Statement of Authorship
All authors contributed equally to data acquisition, manuscript writing, and preparation and approval of final manuscript.