Introduction
Third nerve palsies (TNP), characterized by diplopia, ptosis, ophthalmoplegia, and pupillary dysfunction, is reported to be the most common clinical presentation of posterior communicating artery aneurysms. The risk of TNP in PComm aneurysms is as high as 34–56% Reference Lee, Hayman and Brazis1 The pathophysiology of this phenomenon includes (1) the compressive effect of the aneurysmal outgrowth on the oculomotor nerve and (2) the constant disruptive effect of aneurysmal pulsatility on adjacent cranial nerves. Generally, intracranial saccular aneurysms, if left untreated, can progress over time and may acutely rupture, compromising the quality of life for approximately 35% of patients. Reference Taufique, May and Meyers2 Management of either ruptured or unruptured saccular intracranial aneurysms includes open surgical clipping or endovascular repair (i.e. endosaccular and/or endoluminal approaches). Surgical clipping is believed to alleviate the direct mass effect of aneurysms by obliterating the vascular defect with the added capability of reducing the mass effect on adjacent structures by decompressing the aneurysmal mass. Endovascular approaches such as coiling promote progressive thrombosis of the aneurysm sac, thereby achieving decompression by preventing pulsatile movement within the fundus. While open surgical clipping exposes the patient to the risks associated with craniotomy, coiling has been associated with its own inherent risks, namely the potential to worsen the compressive effects of the aneurysm. Furthermore, arterial pulsatility, primarily at the aneurysmal neck, may result in failure of aneurysm exclusion from the circulation and could potentially lead to coil compaction, aneurysm regrowth, and re-rupture. Reference Turjman, Massoud, Sayre and Viñuela3
The vast majority of patients presenting with a TNP are more likely to have a PComm aneurysm, however, saccular aneurysms involving the basilar apex, SCA, and supraclinoid ICA have also been described in patients presenting with a TNP. Reference Bohnstedt, Ziemba-Davis and Edwards4–Reference Schuss, Güresir, Berkefeld, Seifert and Vatter8 Furthermore, other aneurysm-associated cranial neuropathies have been reported included compression of the optic nerve and trochlear nerve. Reference Mino, Yoshida, Morita and Tominaga6,Reference Engelhardt, Berge, Cuny and Penchet9 The etiology and pathophysiology underlying TNP secondary to PComm aneurysms has not been clearly elucidated, contributing to the uncertainty in favoring one treatment modality over another. The PComm accompanies the oculomotor nerve through its cisternal course; as such, it is thought posterolateral PComm aneurysms cause TNP secondary to a mass effect (Figure 1). Reference Gaberel, Borha, di Palma and Emery10 In fact, the angioarchitecture of the aneurysm has been observed to dictate the increased chance of a complete TNP, especially in aneurysms greater than 7mm in size. Reference Zhong, Zhang and Shen11 However, along its course, the oculomotor nerve passes through and between the posterior cerebral artery (PCA) and the superior cerebellar artery (SCA). Recent reports of successful TNP resolution with endovascular coiling of saccular aneurysms lend support to the pathophysiology of TNP secondary to the transmission of arterial pulsatility, or nerve irritation with edema, rather than an isolated mass effect. Reference Zhong, Zhang and Shen11–Reference Güresir, Schuss, Setzer, Platz, Seifert and Vatter13
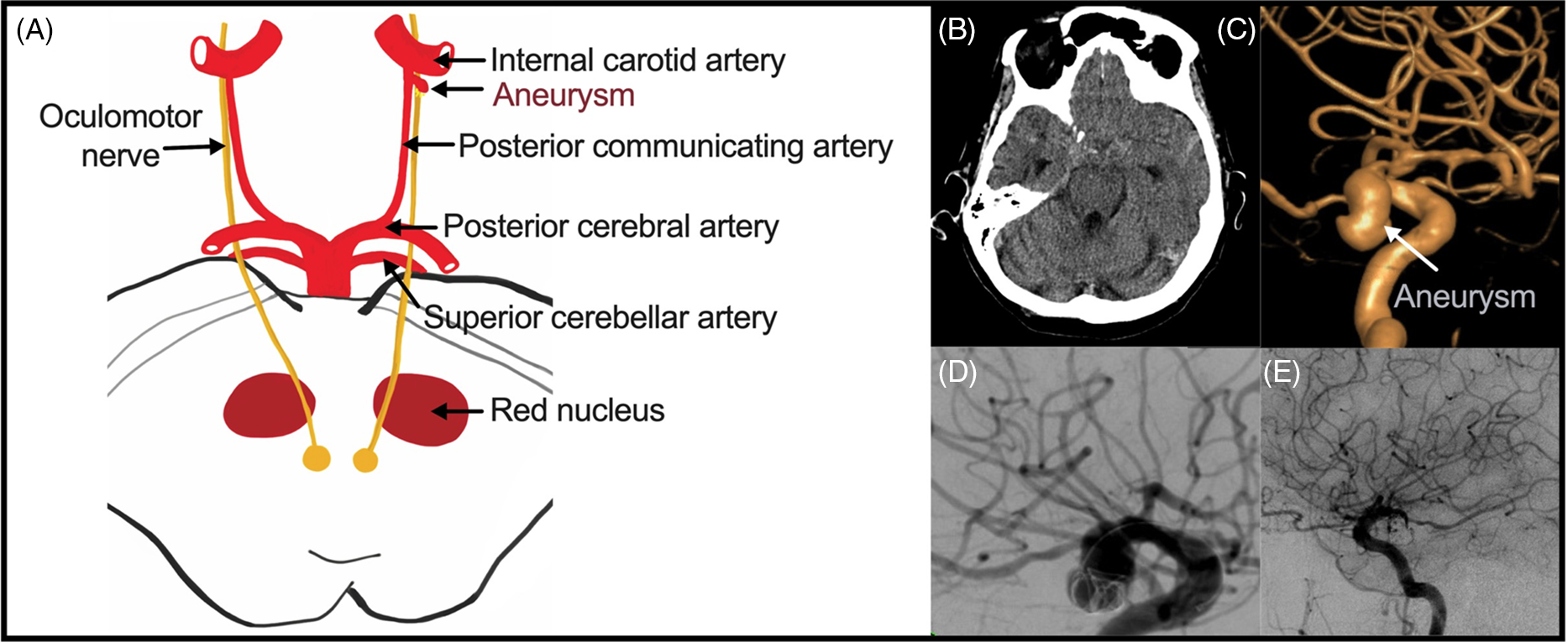
Figure 1: Intracranial aneurysms causing TNP. (A) Anatomical relationship between the oculomotor nerve and the circle of Willis and its branches. (B) Right-sided PComm aneurysm (C) Volume-rendered image of a PComm aneurysm. (D) DSA image demonstrating a coiling of PComm aneurysm. (E) Post-coiling contrast run demonstrating obliteration of a PComm aneurysm with coil embolization.
In this retrospective, single-center study, we analyzed all intracranial aneurysms associated with partial or complete TNP in order to assess the relationship between treatment modality, TNP resolution, and associated predictive factors and compare our findings to the available literature.
Methods
A retrospective cohort study was conducted at a mid-sized Canadian neurosurgical center over a 15-year period (2003–2018). The study was approved by the local university ethics committee. Data were extracted from electronic medical records (Meditech and Sovera) and the Picture Archives and Communication Systems (PACS). To maximize our sample size, all consecutive patients were admitted to the hospital with a Computed Tomography Angiogram (CTA), Magnetic Resonance Angiogram (MRA) or Digitally Subtracted Angiogram (DSA) confirmed intracranial aneurysm who underwent a neurointerventional/neurosurgical procedure by the senior authors were included in the study. Both elective and emergent (i.e. SAH) cases were included for analysis. Complete TNP was defined prior to data extraction as complete ptosis, extraocular muscle palsy, and mydriasis. Partial TNP was any spectrum of deficits that were attributable to the oculomotor nerve that did not comprise the triad of complete TNP. Data were collected using clinical notes from electronic health records at our institution that included copies of all paper charts.
Statistical Analysis
Categorical variables were reported using counts and proportions and compared using the χ2 or Fisher’s test. Continuous variables were reported as mean (SD) and was compared using the independent samples t-test or Mann–Whitney U test as appropriate. For pre and post-intervention analysis, McNemar’s test was employed for paired data. The level of significance was set at 0.05. SPSS software (www.IBM.com) was used for the analysis.
Results
A total of 538 adult patients underwent treatment for at least one intracranial aneurysm at the Hamilton General Hospital between 2003 and 2018. Table 1 summarizes the TNP cohort showing the 538 patients undergoing 574 treatments with only 37 (6.9%) presenting with clinical signs of a partial or complete TNP. The mean age at presentation of intracranial aneurysm-associated TNP-treated endovascularly was 60.1 (SD 11.9) years, the majority were female (91.8%) with half presenting after a subarachnoid hemorrhage (45.9%). The mean size of aneurysm was 9.48 (SD 7.2, range 2.8–31.8) mm. Over half of the cohort harbored a PComm aneurysm-associated TNP (64.9%) but other locations included ICA (29.7%), MCA (2.7%), and basilar apex (2.7%), with 67.6% of TNP-associated aneurysms located on the right side. The number of patients diagnosed with partial TNP was nearly threefold higher than those presenting with a complete TNP.
Table 1: Characteristics of aneurysms associated with TNP undergoing endovascular embolization (n = 37)

Almost the entire cohort of TNP patients was treated with endovascular coiling, with only one patient treated with surgical clipping. Table 2 details the proportion of TNP involvement and surgical intervention received. All 30 patients presenting with a partial TNP underwent endovascular embolization. By contrast, of the eight patients presenting with a complete TNP, one patient underwent surgical clipping while the remaining 7 (87.5%) were treated with coiling (Figure 2). Dichotomizing the SAH and unruptured aneurysm cohorts, the average size of aneurysm (7.07mm vs. 12.7mm), time to presentation (2.5 days vs. 44 days), and time to improvement (65 vs. 198 days) were all higher in the unruptured group. As only one patient with a TNP was surgically clipped with complete recovery of the cranial nerve function, no clinical correlation of TNP recovery and aneurysm procedure was feasible. Given the small cohort, a correlational analysis of time to presentation and recovery was not possible but a trend toward lower likelihood of recovery of TNP with a prolonged time to presentation and time to operative procedure was observed. Table 3 summarizes the trend of the clinical course of the TNP after the intervention. Furthermore, in keeping with previous observations, patients presenting with SAH were less likely to experience TNP recovery after coiling when compared to those presenting with an unruptured aneurysm. In our endovascularly treated cohort, 5/7 (71.4%), 4/10 (40%), and 8/20 (42.9%) of patients with no, partial, and complete TNP recovery, respectively, presented with SAH.
Table 2: Intracranial aneurysm and TNP status

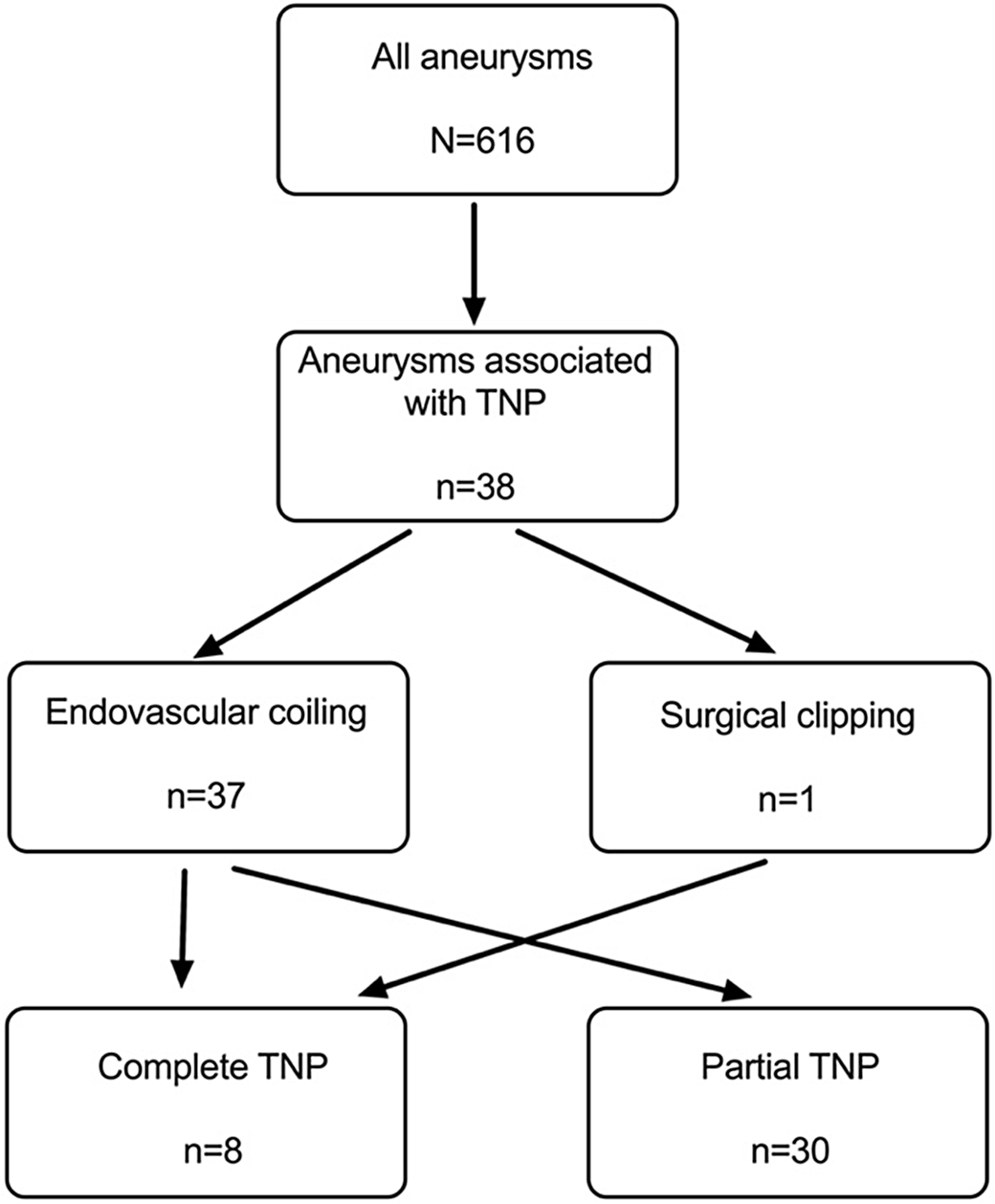
Figure 2: Flow chart of patients with TNP from a single-center cohort.
Table 3: Intracranial aneurysm and TNP status and clinical outcome after endovascular embolization

With respect to the location of aneurysm and TNP recovery, a similar proportion of TNP secondary to ICA or PComm recovered either partially or completely (Table 3). However, a higher proportion of TNP that did not experience any recovery was secondary to PComm (24% vs. 9.1%). Location of aneurysm was not significantly correlated with TNP recovery (p=0.499). Despite the concerns of mass effect of very large aneurysms, no significant correlation was observed between aneurysm size (i.e. aneurysms ≤10mm, 10–19.9mm, ≥20mm) and TNP recovery (p = 0.175, Fisher’s exact) despite imaging findings (Table 4).
Discussion
Oculomotor palsy associated with an intracranial aneurysm was first surgically treated by Walter E Dandy in 1937 with complete resolution of the patient’s “pea-sized” mesially projected ICA aneurysm-associated cranial nerve palsy after 7 months. Reference Dandy14 While surgical clipping was historically the mainstay of intracranial aneurysm management, there have been remarkable advances in endovascular approaches since the publication of the International Subarachnoid Aneurysm Trial (ISAT), resulting in a significant reduction in the frequency of surgical clipping. While the exact pathomechanism of aneurysm-associated TNP has not been fully elucidated, our single-center experience suggests a role for the pulsatility theory, as we observed a trend toward resolution of TNP of both PComm and non-PComm aneurysms after endovascular aneurysm coiling, especially for cases without accompanying SAH (Table 2). As there was only one patient presenting with a TNP with subsequent clipping of the associated aneurysm, we were unfortunately unable to compare the two modalities for the efficacy of TNP recovery after treatment (i.e. coiling vs. clipping). Despite current limitations in published data describing the safety and efficacy of endovascular coiling of an aneurysm causing a cranial nerve palsy, there seems to be a general trend toward resolution suggesting the coil mass does not cause a permanent mass effect on the oculomotor nerve Reference Mino, Yoshida, Morita and Tominaga6,Reference Schuss, Güresir, Berkefeld, Seifert and Vatter8,Reference Engelhardt, Berge, Cuny and Penchet9,Reference Zhong, Zhang and Shen11–Reference Güresir, Schuss, Setzer, Platz, Seifert and Vatter13,Reference Ahn, Han and Yoon15–Reference Tan, Huang, Zhang, Liu, Li and Wang22 (Table 3). Further technological advances have broadened endovascular treatment options with endoluminal and endosaccular devices for treating intracranial aneurysms with complex angioarchitecture, thus the trend toward endovascular management will continue to evolve, and in many centers, it has surpassed open surgical treatment.
Table 4: Studies reviewing TNP recovery after intracranial aneurysm treatment

* Unreported for 11 patient.
** For ruptured aneurysms, no difference w/unruptured aneurysms.
*** Pre-operative TNP status for 1 patient unknown.
The historical evidence for clipping an intracranial aneurysm with associated cranial nerve palsy has almost exclusively been small, retrospective single-center studies, spanning over several years with varying clinician skills and availability of treatment adjuncts (Table 3). Gaberel et al. (2016) demonstrated no significant difference in a meta-analysis of 297 patients (RR = 1.48, 95% confidence interval 0.95–2.29, p = 0.08), however, these results are limited by the inconsistent follow-up times across the included studies. Reference Zheng, Dong and Xia23 In a subsequent meta-analysis by Zheng et al. that included two additional studies, there was a significant advantage of clipping in achieving full TNP recovery (OR = 4.44, CI interval 1.66–11.84, p = 0.0003). Reference Gaberel, Borha, di Palma and Emery10 The authors in this meta-analysis concluded clipping is associated with a greater rate of total recovery within a subgroup analysis of ruptured aneurysms – the advantage was attributed to the additional mass effect placed on the oculomotor nerve by the hematoma which was decompressed at the time of surgical clipping. Reference Gaberel, Borha, di Palma and Emery10,Reference Zheng, Dong and Xia23,Reference McCracken, Lovasik and McCracken24 While there are concerns that potential nerve traction could worsen TNP during surgical clipping, the physical decompression of the aneurysmal sac has the added benefit of reducing both pulsatility and mass effect. Reference Güresir, Schuss, Setzer, Platz, Seifert and Vatter13 During surgery, nerve adhesions can also be lysed to potentially further improve TNP outcomes.
To further support our hypothesis of aneurysmal pulsatility-associated TNP, there are limited large-scale comparative studies analyzing differences in outcome between surgical the intervention (i.e. clipping vs. coiling) with no definitive conclusions drawn due to a small number of cases (Table 4). Overall, these studies suggest greater rates of TNP recovery with clipping compared to coiling. Reference Signorelli, Pop and Ganau12,Reference Chen, Amin-Hanjani, Albuquerque, McDougall, Zabramski and Spetzler17,Reference Khan, Agrawal and Hailey18,Reference McCracken, Lovasik and McCracken24,Reference Kwon, Huh, Kim and Lee25 However, in the absence of randomized trials, surgical clipping was and continues to be broadly regarded as a reliable approach to aneurysm-induced TNP with single-center rates of complete resolution ranging from 58.0% to 92.3%. Reference Gaberel, Borha, di Palma and Emery10,Reference Park, Kang and Chun26 Predictably, patients selected for endovascular treatment tended to be poorer surgical candidates, as they were older and presented with more comorbidities (e.g. diabetes, hypertension, hyperlipidemia). Reference Zhong, Zhang and Shen11 While our study cannot offer direct comparisons of these modalities, 20 of the 37 (54.1%) patients treated with endovascular coiling experienced complete TNP recovery. The rate of TNP recovery (81.6%) after coiling in our patients was similar to other published single-centered cases Reference Mino, Yoshida, Morita and Tominaga6,Reference Schuss, Güresir, Berkefeld, Seifert and Vatter8,Reference Zhong, Zhang and Shen11,Reference Ahn, Han and Yoon15,Reference Chen, Amin-Hanjani, Albuquerque, McDougall, Zabramski and Spetzler17–Reference Tan, Huang, Zhang, Liu, Li and Wang22 and significantly greater than the 42.5% reported in the meta-analysis data. Reference Gaberel, Borha, di Palma and Emery10 The significance of timing in endovascular therapy also remains ambiguous, with suggestions that treatment within a week may improve recovery rates. Reference Zu, Liu and Wang27 The duration of preoperative TNP is thought to be an important prognostic factor; in its initial stages, TNP secondary to vessel conflict is likely a result of neuropraxia, while axonal degeneration and ischemia follow within months. Reference Mansour, Kamel and Kelleher28 Furthermore, recovery is more likely with incomplete preoperative TNP (p = 0.03, OR = 4.2), Reference Güresir, Schuss, Setzer, Platz, Seifert and Vatter13 which may reflect the degree and subsequently reversibility of nerve damage. Of note, recovery rates with endovascular coiling in single-arm studies reported lower rates of TNP recovery when compared to surgical clipping (range 35.0–61.8%), Reference Gaberel, Borha, di Palma and Emery10,Reference Zhong, Zhang and Shen11,Reference Su, Shi, Ge and Li21 despite greater rates reported for shorter time to treatment, smaller aneurysm size, and stent use. This emphasizes the concept that recovery of TNP after aneurysm treatment is multifaceted and multiple factors including and not limited to the duration of TNP, size of aneurysm, past medical history, SAH presentation have to be taken into account when determining the safest and effective management modality. Our results are unexpected, given the longer mean time to treatment (47.1 days vs. 10.5 days) and larger mean aneurysm size (8.62 mm and 7.40 mm), with comparable age and follow-up period in the endovascularly treated group.
A significant limitation to meta-analyses of TNP recovery and intracranial aneurysm treatment, in general, is the definition of a complete and partial TNP. For example, one study considered complete TNP was the presence of concurrent ophthalmoplegia, ptosis, and diplopia, Reference Engelhardt, Berge, Cuny and Penchet9 while another study required ptosis, extraocular muscle palsy, and mydriasis for the same diagnosis. Reference Güresir, Schuss, Setzer, Platz, Seifert and Vatter13 Furthermore, individual studies were found to be highly variable in their time to treatment, and follow-up duration. Despite lower rates of TNP recovery in the literature, recovery nonetheless was observed in endovascularly coiled patients suggesting the pulsatility theory of neurapraxia may be the predominant pathomechanism by which the TNP develops as opposed to the compressive mass effect of the aneurysm on the oculomotor nerve. Specific to our study, the intrinsic selection bias inherent to all retrospective studies, there are several limitations to our findings (e.g. small number of clipped patients not facilitating a comparative analysis of TNP recovery, limited objective documentation of definition of TNP, and poor granularity of data regarding assessment of recovery).
Conclusion
In our intracranial aneurysm-associated TNP cohort, the vast majority of the patients in this study experienced either partial or complete TNP resolution (31/38; 81.6%), establishing the feasibility of the endovascular approach for TNP secondary to PComm aneurysms. With the fast pace of endovascular technological advances in aneurysm treatment and expanded use of flow diverters in addition to a consistent diagnostic definition of TNP is required to resolve the question of the efficacy of TNP resolution after endovascular coil embolization of TNP-associated intracranial aneurysms. Our findings support the role of pulsatility-induced neurapraxia of the oculomotor nerve causing aneurysm-induced TNP and provide evidence for the utility of endovascular repair of this entity.
Acknowledgments
We recognize all the nurses and residents who have diligently documented the data for the patients included in this study. We have no financial disclosures.
Disclosures
The authors have no conflicts of interest to declare.
Statement of Authorship
MKS, BvA, TG, PK, FF, RL, AA conceptualized the research question. MKS, AP, KH, AAA, YJ extracted that data from the databases. AM provided administrative support. MKS, BvA, AP, AAA, DK, FF analyzed and interpreted the data. MKS wrote the manuscript with significant revisions contributed by BvA, AP, YJ, and FF. BvA, TG, PK, and FF supervised the study. All authors reviewed the results and commented on the manuscript.








