Glioblastoma is an incurable tumor with a median expected survival of less than 2 years from diagnosis. The most important goal of therapy remains the optimization of the patient’s quality of life. Dysgeusia is an alteration of sensation where normal taste is perceived as bad or unpleasant. It is a frequent, yet underreported adverse effect of systemic chemotherapy that may have a significant impact on a patient’s nutritional status and quality of life.Reference Ponticelli, Clari and Frigerio1 Over the last years, we managed two patients who received chemotherapy for glioblastoma and developed such a severe dysgeusia that treatment discontinuation was considered. Symptom relief was achieved in both cases using gabapentin. This is the first report of gabapentin use for chemotherapy-induced dysgeusia.
The first patient was a 60-year-old man with a left frontal operculum glioblastoma, IDH wild type. He underwent a craniotomy with gross total resection of the tumor, followed by combined radiotherapy (60 Gy in 30 fractions) and temozolomide (75 mg/m2). Tumor recurrence occurred immediately after the end of radiotherapy (Figure 1, case 1) and a second surgical resection was performed without complication, after which the patient was enrolled in a trial of cerebral intra-arterial chemotherapy (CIAC) using carboplatin (748 mg monthly). The patient first complained of taste disturbance (all flavors) at 5 months from the beginning of CIAC. In retrospect, consumption of sweets had been making him nauseous since the first dose of carboplatin and a 3% decrease in weight was noted in the 6 weeks following the first CIAC treatment (Figure 2). Standard nutritional counseling was performed (Supplementary Table 1) and nutritional supplementation was attempted, although the patient did not tolerate the taste of any supplements except Beneprotein. At 7 months, the patient had lost 10% of his body weight and enteral feeding was offered and declined by the patient. At 8 months, the patient was admitted for a global physical deterioration and his next CIAC cycle was cancelled. While the tumor had been radiologically stable since the surgery, the patient had lost 11% of his pre-CIAC body weight and had an estimated food intake of less than 500 kcal/day. The patient reported severe dysgeusia and the tasting of any food now triggered nauseas. His quality of life was at its lowest. Given the failure of conventional nutritional counseling, the patient was started on zinc supplements (50 mg daily). The patient reported marginal improvement, but still remained severely disabled by his dysgeusia. After a thorough discussion within the medical team, the patient was then prescribed gabapentin 100 mg thrice daily. Within 3 days, he noted some improvement, increased his food intake, and could be discharged home. Two weeks later, his dysgeusia had almost completely disappeared and his appetite was back. He had regained 2.8 kg (5%) and reported that his quality of life was greatly improved. He had no side effect from the gabapentin. CIAC was resumed for two cycles with no further deterioration in taste. Tumor progression occurred during the next cycle and the patient died at 10 months from the first CIAC, or 12 months from the initial diagnosis.

Figure 1: MRI of both cases at the time of chemotherapy initiation.
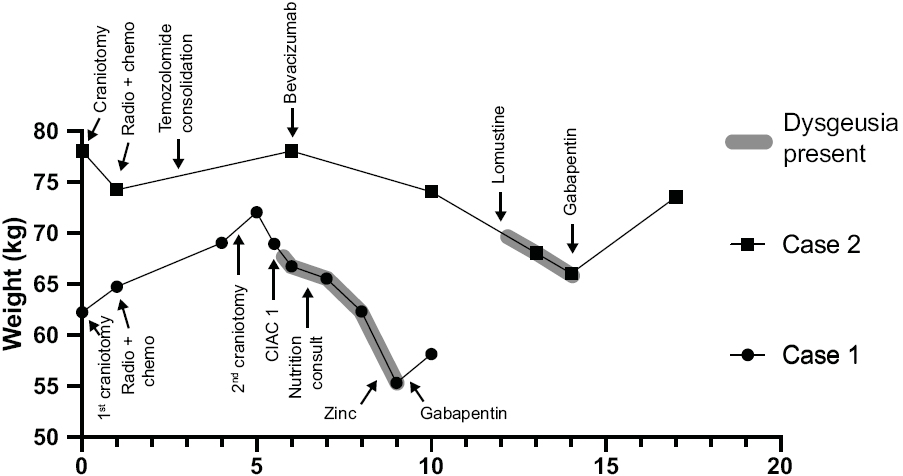
Figure 2: Weight evolution relative to medical and nutritional interventions in both cases.
The second case is a 66-year-old man with a right posterior frontal corona radiata glioblastoma, IDH wild type (Figure 1, case 2). He underwent a subtotal resection followed by combined radiotherapy (60 Gy in 30 fractions) and temozolomide (75 mg/m2). Three cycles of temozolomide consolidation (200 mg/m2 daily for 5 days every 28 days) were completed until progression occurred, at which point temezolomide was stopped and a bevacizumab regimen begun (10 mg/kg every 2 weeks). The lesion was controlled for an additional 6 months, until further progression occurred at 12 months from the initial diagnosis. Lomustine (200 mg every 6 weeks) was then added. The patient started complaining about dysgeusia within 1 month of the first lomustine dose. Three weeks after the second dose, his dysgeusia was so severe that he refused to eat, leading to a 15% weight lost. Given the anecdotal success experienced with the first case, gabapentin was introduced (100 mg thrice daily for 7 days, then 300 mg thrice daily). Within 1 week, his taste disturbance improved and he started to regain appetite and energy. His weight increased by 11%. We are now at 2 years from the initial diagnosis with the tumor and associated edema currently controlled by bevacizumab and lomustine. The patient has been on gabapentin for 8 months with no recurrence of dysgeusia despite continuation of the causative agent.
The two cases presented above constitute the first detailed report of dysgeusia occurring in the setting of glioblastoma treatment. We successfully achieved symptomatic control in both cases using gabapentin. While central processes such as epilepsyReference Heckmann, Heckmann, Lang and Hummel2 or even fibrillary astrocytomaReference Mignogna, Adamo, Falleti and Fortuna3 have been shown to cause dysgeusia, our patients’ symptoms were not intermittent (as would be expected from epilepsy) and did not correlate with radiological changes in the lesion. There were no brainstem, hypothalamic, or pituitary involvement in either case, and dysgeusia was not associated with surgical complications (such as CSF leaks or rhinorrhea). Rather, the timing of the symptom occurrence and resolution in our cases suggest that dysgeusia was a side effect of their chemotherapy regimen (carboplatin or lomustine). Indeed, dysgeusia is an often overlooked complication of chemotherapyReference Ponticelli, Clari and Frigerio1 While we could not find specific data on temozolomide or lomustine, taste alterations have been reported to occur in up to 77% of oncological patients undergoing systemic treatment with various other molecules (etoposide, leucovorin, irinotecan, and platinum-based agents).Reference Lindley, McCune and Thomason4–Reference Zabernigg, Gamper and Giesinger6 Symptoms, once installed, usually persist for weeks or even months before subsiding after the cessation of the causing chemotherapeutic agent. In our experience, this side effect is likely underreported and most patients will not mention it unless specifically asked.
The pathophysiology of chemotherapy-associated dysgeusia is poorly understood. One hypothesis is that systematic chemotherapy could deplete body zinc reserve through chelation. It has been hypothesized that zinc plays an important role in nerve conduction and in gustatory cell surface chemoreceptors’ functions. A systematic review of possible interventions for taste disturbanceReference Kumbargere Nagraj, George, Shetty, Levenson, Ferraiolo and Shrestha7 found very low-quality evidence to conclude on the role of zinc supplementation in taste disorders, but moderate ones for improvement of overall taste thresholds. In studies where zinc was found to be helpful, supplements were given prior to chemotherapeutic cycles and continued throughout the therapeutic course. The beneficial effects of zinc, although still controversial, could involve a quicker recovery of taste perception in the following weeks after cessation of chemotherapy. In any case, the benefits of zinc are usually through an impact on the taste threshold rather than taste quality.Reference Kumbargere Nagraj, George, Shetty, Levenson, Ferraiolo and Shrestha7
Given that our patients complained of a taste disturbance and not ageusia, we did not believe zinc would resolve their symptoms. After trying zinc in the first case with little success, we found a case report by Devere et al. where a patient affected by dygeusia and dysosmia was successfully treated with gabapentin.Reference Devere8 We therefore tried this strategy, approaching our two patients as having a deafferentation syndrome. The hypothesis was that the chemotherapy could have inhibited gustatory cells turnover and function,Reference Berteretche, Dalix, d’Ornano, Bellisle, Khayat and Faurion9 leaving first-order gustatory neurons without their normal afferent signal from taste buds. We postulated that our patients’ dysgeusia was the taste equivalent of a standard neuropathic pain disorder. While no formal testing (EMG, somatosensory evoked potentials, etc.) was performed to confirm this hypothesis, the good response to gabapentin certainly supports a neurological component to the patient’s symptoms. In the end, addressing these patients’ dysgeusia allowed resumption of their chemotherapy, weight gain, and, most importantly, improvement in their quality of life.
We reported two cases where gabapentin was used to successfully manage disabling dysgeusia in patients undergoing chemotherapy for progressive glioblastoma. The resolution of dysgeusia had a significant beneficial impact on nutritional status and quality of life, which was maintained until last follow-up and without significant side effects. Given the high prevalence of dysgeusia in the cancer population, its occurrence should probably be specifically sought in all patients. When it occurs, formal nutritional counseling and diet optimization should be tried first (Supplementary Table 1), with or without zinc supplementation. If the symptom persists, our results suggest that a gabapentin trial might be effective. This interesting finding should be further explored by other groups and validated in the setting of a formal clinical trial.
Disclosure
The authors have no conflicts of interest to report.
Statement of authorship
DF and CIM treated the patients described herein. KT, CJT, and CIM collected the data. CIM produced the figures. KT wrote the first draft of the manuscript. CJT, CIM, and DF critically reviewed and improved the final draft. All authors approved the final version and agree to submit to CJNS.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/cjn.2020.115.




