Introduction
Delayed cerebral ischemia (DCI) is a complication of aneurysmal subarachnoid hemorrhage (aSAH) and is associated with significant morbidity and mortality. Reference Ferguson and Macdonald1,Reference Rosengart, Schultheiss, Tolentino and Macdonald2 Among patients who survive initial aneurysm rupture, ∼20%–30% develop DCI. Reference Ferguson and Macdonald1,Reference Connolly, Rabinstein and Carhuapoma3,Reference Dorhout Mees, Kerr, Rinkel, Algra and Molyneux4 The pathogenesis of DCI is not fully understood; mechanistic hypotheses include impaired autoregulation, vasospasm, microcirculatory dysfunction, cortical spreading depression, inflammation, and delayed cell apoptosis. Reference Findlay, Nisar and Darsaut5–Reference Schoch, Regel, Wichert, Gasser, Volbracht and Stolke7 Randomized clinical trial data and strong consensus guidelines are lacking for many aSAH-related interventions, including those related to the prevention and treatment of DCI. Reference Connolly, Rabinstein and Carhuapoma3
Preventative therapies for DCI include the optimization of blood volume and cardiac performance. Reference Findlay, Nisar and Darsaut5,Reference Francoeur and Mayer8 Oral nimodipine is a dihydropyridine calcium channel blocker that is typically used to reduce the morbidity associated with DCI. Reference Connolly, Rabinstein and Carhuapoma3,Reference Pickard, Murray and Illingworth9 Serial clinical examinations, Doppler ultrasound, and computed tomography (CT) angiography are utilized for monitoring and screening for DCI. Clinical trials for numerous pharmacologic agents have all failed to show benefit in preventing or treating DCI. Reference Francoeur and Mayer8,Reference Kirkpatrick, Turner, Smith, Hutchinson and Murray10–Reference Macdonald, Higashida and Keller12 Milrinone is an inotropic phosphodiesterase-3 (PDE-3) inhibitor that has also been studied for its role in the treatment of DCI. It acts as a potent vasodilator and may exert anti-inflammatory effects. Reference Gong, Lin, Lu and Zheng13 Lannes et al. reviewed studies of milrinone use in patients at risk of or suffering from DCI after aSAH, Reference Lannes, Zeiler, Guichon and Teitelbaum14 and more recently, Abdulhasan et al. published a large retrospective study based on the Montreal Neurological Hospital protocol that found milrinone use both safe and effective in the management of DCI. Reference Abulhasan, Jimenez, Teitelbaum, Simoneau and Angle15,Reference Lannes, Teitelbaum, del Pilar Cortés, Cardoso and Angle16 While the evidence for milrinone use in this patient population remains low, it is commonly used as a rescue therapy for the treatment of DCI.
Unfortunately, high-quality evidence to guide the prevention, monitoring, and management of DCI is lacking. The Canadian Neurosurgery Resident Research Collaborative is comprised of resident neurosurgeons in a unique position to capture multicenter data and appraise differing national practice patterns. In this context, the objective of this study was to evaluate the practice patterns of Canadian physicians regarding the prevention, diagnosis, and treatment of DCI after aSAH. Understanding current practice patterns may inform the development of future targeted prospective clinical trials and allow development of national standards in the definition, identification, and treatment of DCI.
Methods
Study Population
We conducted a cross-sectional survey of Canadian physicians who diagnose, monitor, and manage aSAH. Those eligible to complete the questionnaire included neurosurgeons, intensivists, neurologists, neuro-intensivists, vascular surgeons, and interventional radiologists. Residents and fellows in the aforementioned specialties were also eligible. The survey was distributed to the Canadian members of the Neurocritical Care Society (NCS) and Canadian Neurosurgical Society mailing lists. The survey was also electronically distributed via members of the Canadian Neurosurgery Resident Research Collaborative (CNRC) to practitioners at Canadian university-affiliated teaching hospitals.
Survey Development
A narrative review (MEDLINE, Embase, PubMed) of the relevant literature was conducted in order to identify DCI management domains lacking scientific consensus and requiring further investigation. A 19-question survey was developed to highlight several domains with respect to the management of DCI following aSAH: diagnosis, monitoring, prevention, and treatment. The aforementioned domains were subdivided further according to the available evidence and consensus, or lack thereof. We adapted a previously reported method of survey testing. Reference Turgeon, Lauzier and Burns17 A multi-step approach was taken to assess our survey for validity (face and content), feasibility, and reliability. First, two co-investigators (ME and JT) provided a draft of the questionnaire to the participating investigator members of the CNRC. The survey was assessed for redundancy and clarity. Second, content experts (e.g., neurosurgeons, neuro-interventionists) assessed the survey for face and content validity. The survey was then revised and finalized (see Appendix 1).
Survey Administration
Survey completion was anonymous and voluntary. Results were collected via Survey Monkey, a user-friendly, online (http://www.Survey Monkey.com), electronic survey platform. No paper format of the survey was distributed. No financial incentive was provided for completing the survey. Members of the CNRC, content experts, and all invited clinicians were sent a personalized electronic mail allowing them to access the online survey. The eligibility of respondents was verified at the beginning of the survey; if the respondents indicated answered that they do not manage DCI, the questionnaire closed. If the respondent answered more than 80% of the questionnaire, it was considered complete (American Association for Public Opinion Research, 7th Edition, 2011).[MM5] Survey completion was voluntary and anonymous.
A follow-up email was sent out at the 3rd and 5th weeks to non-responders. Members of the CNRC provided a verbal reminder at the 4th and 6th weeks to neurosurgical and neuro-intensivist attendings and fellows at their hospitals. This approach ensured access to nearly all university-affiliated teaching hospitals in Canada.
Statistical Analysis
Descriptive statistics (proportions with 95% confidence intervals) were used to report results. Differences across response distributions were assessed using either the chi-square or Fisher’s exact test. Observations with missing data were excluded from statistical testing. Microsoft Excel and GraphPad Prism software were used for statistical analysis and figure generation.
Results
Respondent Demographics
The response rate after all mailings was 129/340 (38%). The majority of respondents were neurosurgeons (44.4%) and intensivists (35.7%) with subspecialty training in either neurosurgery or neurology (Figure 1A). Approximately 40% of respondents were attending staff with <10 years in practice; 31.8% of respondents reported practicing for >10 years, and 19.7% of respondents were residents (Figure 1B). Seventy-seven percent of respondents practiced in institutions where they care for >35 cases of aSAH per year (Figure 1D).

Figure 1: Demographic data shown as percentage of total respondents. (A). Respondent subspecialty. (B). Respondent level of experience. (C). Perception of the frequency of delayed cerebral ischemia (DCI) after aneurysmal subarachnoid hemorrhage (aSAH). (D). Number of aSAH cases observed per year.
Diagnosis and Definition of DCI
The majority (98.1%) of respondents felt that <50% of aSAH admissions experience DCI (Figure 1C). Within their own facility, 45.7% of respondents reported “lacking a clear definition of DCI” and 19.4% were unsure of their institutional definition of DCI (Figure 2A). Free-text responses were provided for the source of the institutional definition of DCI. Of 12 responses, six (50%) used the 2012 AHA/ASA guidelines, Reference Connolly, Rabinstein and Carhuapoma3 three (25%) defined DCI as a clinical and imaging concordance with neurological deficit, one (8.3%) defined any new neurological deficit as DCI, one (8.3%) used the REACT study protocol (ClinicalTrials.gov Identifier: NCT03585270), and one (8.3%) defined DCI as a diagnosis of exclusion (Figure 2).
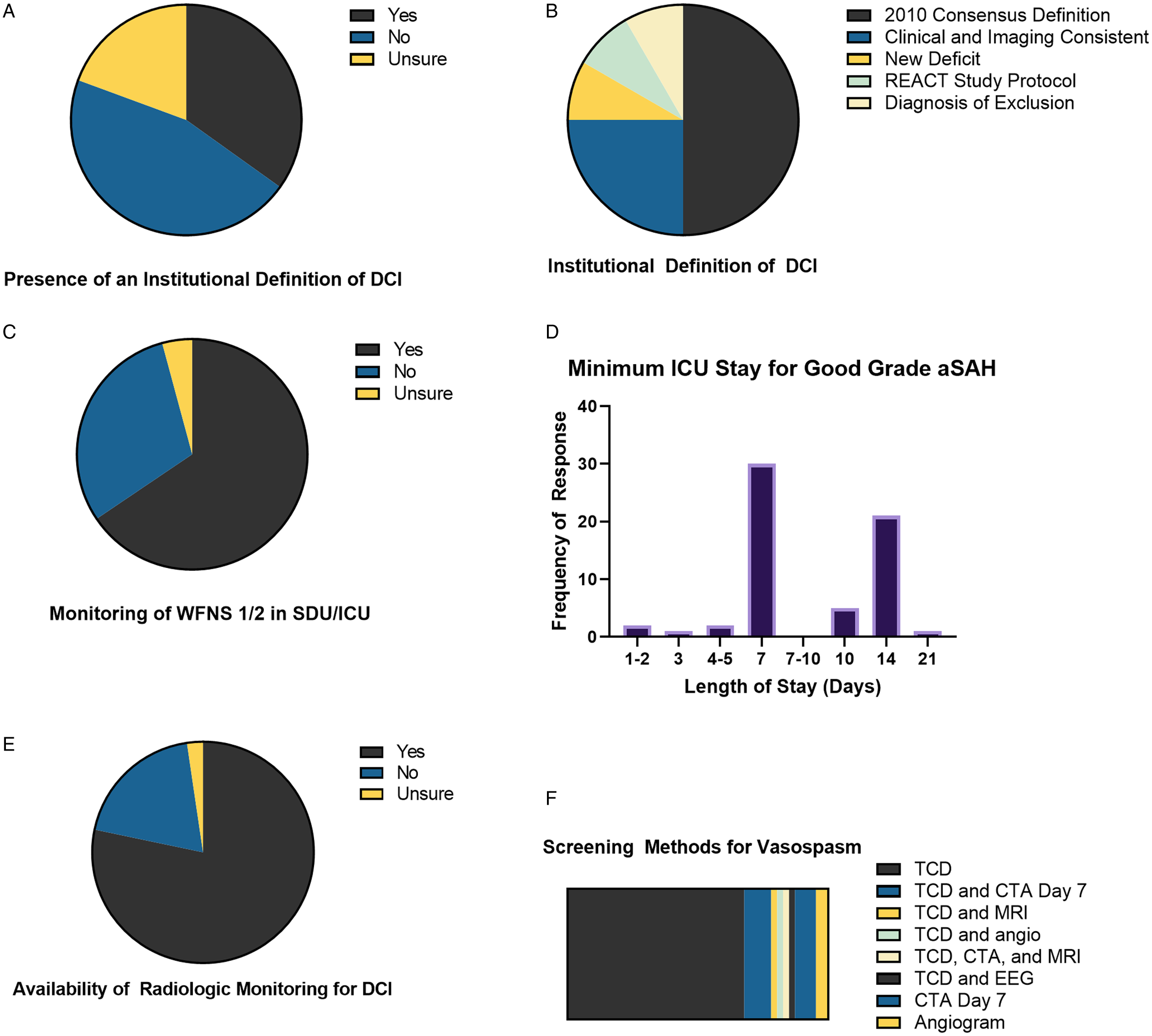
Figure 2: Institutional definitions and monitoring of delayed cerebral ischemia (DCI). (A). Awareness of an institutional definition of DCI. (B). Source for institutional definitions of DCI. (C). Provision of step-down unit (SDU) or intensive care unit (ICU) monitoring for low World Federation of Neurologic Surgeons (WFNS) score patients. (D). Minimum stay in ICU setting for aneurysmal subarachnoid hemorrhage (aSAH) patients with good neurological grade (WFNS Grade 1-2). (E). Availability of radiological monitoring for DCI after aSAH. (F). Screening method utilized to monitor for DCI.
Monitoring for DCI
Over half of respondents (50.4%) reported monitoring of World Federation of Neurological Surgeons (WFNS) Grade 1-2 aSAH patients in either a neurological ICU or neurological step-down unit (Figure 2C). Most respondents recommended monitoring patients with aSAH in an ICU for either 7 or 14 days (Figure 2D). Respondents reported multimodality monitoring of DCI in 40.3% respondents, 19.4% reported use of no tests to monitor DCI post-aSAH, and 2.3% were unsure if their institutions monitor for DCI post-aSAH (Figure 2E). Eighty-seven respondents described their choice of screening modalities. The majority (67.8%) employed transcranial Dopplers (TCDs) and the remainder utilized CTA or combinations of TCD with other radiographic imaging modalities (Figure 2F).
Prevention of DCI
Most respondents (74.4%) attempt to prevent DCI (74.4%) (Figure 3A). Sixty-five respondents gave free-text answers regarding use of DCI prophylaxis. Respondents most commonly used nimodipine alone (46%) or in combination with therapies targeting fluid balance (Figure 3B). Free-text responses indicate that 85.7% of respondents used nimodipine. Evidence used to guide the method of DCI prophylaxis was listed as the 1989 BRANT trial by 35/82 (42.6%), Reference Pickard, Murray and Illingworth9 no evidence used by 10/82 (12.2%), no specific evidence listed despite response by 10/82 (12.2%), NCS guidelines by 9/82 (11.0%), Reference Diringer, Bleck and Claude Hemphill18 American Heart Association/American Stroke Association (AHA/ASA) guidelines by 8/82 (9.8%), Reference Connolly, Rabinstein and Carhuapoma3 the 2007 nimodipine Cochrane review by 5/82 (6.0%), Reference Dorhout Mees, Rinkel and Feigin19 the Montreal Neurological Institute (MNI) Protocol by 3/82 (3.7%), Reference Lannes, Teitelbaum, del Pilar Cortés, Cardoso and Angle16 and more recent trials such as REACT (ClinicalTrials.gov Identifier: NCT03585270) or NEWTON-2 by 2/82 (2.4%) (Figure 3C). Reference Carlson, Hänggi and Wong20

Figure 3: Prophylaxis for delayed cerebral ischemia (DCI). (A). Provision of prophylaxis to prevent DCI. (B). Modalities used for prophylaxis against DCI. (C). Evidence identified in support of prophylaxis methods.
Treatment of DCI
Most respondents (57.4%) indicated their institution has a standardized protocol for management of DCI, whereas 34.9% and 7.8% reported that their institutions do not have a standardized protocol or were unsure if a protocol exists, respectively (Figure 4A). Following failure of first-line therapy for DCI, 55.0% of respondents reported use of a standardized protocol for second-line management, whereas 35.7% and 9.3% stated there was no protocol or were unsure of further management of DCI refractory to first-line therapy, respectively (Figure 4B).

Figure 4: Treatment for delayed cerebral ischemia (DCI). (A). Availability of institutional standard protocol for treatment of DCI. (B). Availability of an institutional protocol for DCI first-line therapy failure for DCI. (C). First-line treatment modalities used in centers with and without DCI protocol. (D). Treatment trigger modality for DCI. (E). Evidence used to guide management of DCI.
Eighteen respondents described their treatment protocol. Of these, seven (38.9%) gave IV fluid bolus followed by vasopressor, five (7.8%) induced hypertension followed by use of an intra-arterial calcium channel blocker, three (16.7%) followed the MNI protocol, Reference Lannes, Teitelbaum, del Pilar Cortés, Cardoso and Angle16 two (11.1%) initiated ICU transfer but did not specify other management, and one (5.6%) used nimodipine. For those centers without protocols, 49 respondents detailed their management. The most common choices were fluids and vasopressors in combination (39.8%), followed by vasopressors (20.4%) or intravenous fluids (16.3%) alone (Figure 4C).
Initiation of first-line hyperdynamic therapy was reported in the setting of clinical neurological deficits only (22.5%), neurological deficits and radiographic vasospasm on CTA (24.8%), deficits and vasospasm on TCDs (14.0%), and deficits with no response to fluid resuscitation (7.0%) (Figure 5A). Forty-six respondents provided a free-text response regarding the criteria for initiating DCI treatment. Of these, the majority (59%) used neurological deterioration as a trigger or both clinical and radiological change together (23.9%) (Figure 4D).

Figure 5: Identification and treatment of delayed cerebral ischemia (DCI). (A). Initiation of hyperdynamic therapy for DCI. (B). Percent of patients meeting definition of DCI and requiring IV milrinone infusion. (C). Percent patients meeting definition of DCI and requiring chemical angioplasty. (D). Percentage of patients meeting definition of DCI and requiring physical angioplasty. (E). Second-line treatment options reported. (F). Starting dose of intravenous milrinone infusion for centers employing its use.
A total of 63 respondents cited the evidence used to guide their first-line management choice. Of these, 19 (30.2%) used no evidence, 15 (23.8%) used the MNI Protocol, Reference Lannes, Teitelbaum, del Pilar Cortés, Cardoso and Angle16 12 (19.0%) used the AHA guidelines, Reference Connolly, Rabinstein and Carhuapoma3 8 (12.7%) used case series evidence, 6 (9.5%) used the NCS guidelines, Reference Diringer, Bleck and Claude Hemphill18 5 (7.9%) cited Gathier et al. published in Stroke (i.e., induced hypertension), Reference Gathier, van den Bergh and van der Jagt21 2 (3.2%) cited other angioplasty literature, and 1 (1.6%) cited the British Nimodipine Trial (Figure 4E). Reference Pickard, Murray and Illingworth9
The order and indications for the use of subsequent therapies varied widely. IV milrinone was used by 71% of respondents. Forty-seven percent of respondents estimated using it in less than 25% of DCI patients, while others (13%) had a more liberal usage, prescribing it in 75%–99% of patients that develop DCI (Figure 5B). Ninety-six percent of respondents reported using chemical angioplasty in at least some cases of DCI. Most (41%) reported using it in less than 25%, 11% used it in 75%–99%, and 8% reported using it in all instances of DCI, respectively (Figure 5C). Seventy-three percent of respondents used physical angioplasty in 0%–25% of DCI patients, while 13% and 4% of respondents used it in 50%–75% and 100% of patients with DCI, respectively (Figure 5D). Free-text responses were given by 16 respondents detailing second-line therapy. Of these, 12 (75%) used intra-arterial therapy, two (12.5%) used milrinone, one (6.3%) used the MNI Protocol, Reference Lannes, Teitelbaum, del Pilar Cortés, Cardoso and Angle16 and one (6.3%) used vasopressors (Figure 5E). In terms of milrinone starting dose, 28 respondents provided free-text responses. There was substantial variation among answers. The most common doses were 0.5 mcg/kg/min (35.7%) and 0.25 mc/kg/min (32.1%) (Figure 5F).
Discussion
This study describes the results of a cross-sectional survey of Canadian subspecialist physicians, describing practice patterns for the prevention, monitoring, and management of DCI following aSAH. We identified several areas of agreement across respondents. These included the need for intensive care unit (ICU) monitoring, use of clinical and radiographic monitoring modalities, and the use of prophylaxis for the prevention of DCI. The significance of this work relates to several inconsistencies identified across responses. For example, the indication for starting hyperdynamic therapy varied significantly. There was discrepancy in the proportion of clinicians that reported using IV milrinone, IA vasodilators, or physical angioplasty for the treatment of DCI. Furthermore, our findings highlight deficiencies regarding the use of standardized definitions for DCI and protocols for the work-up and management of this known complication. This is not overly surprising, as the most recent guidelines for the diagnosis and management of DCI after aSAH consisted primarily of class 2 and 3 evidence (for a summary, see Appendix 1). Reference Connolly, Rabinstein and Carhuapoma3 Herein, we discuss numerous avenues for future research.
Prophylaxis for DCI
Despite extensive basic research to date, no effective preventive therapy for DCI is widely available. Most survey respondents indicated they use oral nimodipine as chemoprophylaxis in patients with aSAH. This is not surprising, given that use of oral nimodipine is supported by Class I evidence as it improves neurological outcomes but not cerebral vasospasm after aSAH. Reference Pickard, Murray and Illingworth9,Reference Macdonald, Higashida and Keller12,Reference Shen, Pan, Fan, Xiong and Zhan22 Most respondents reported their decision to use nimodipine was supported by the (British) Nimodipine Trial. Reference Pickard, Murray and Illingworth9 The second most frequently reported prophylaxis was a combination of nimodipine and intravenous fluids to maintain normovolemia. This finding is also supported by AHA/ASA Guidelines for the Management of aSAH which recommend maintenance of euvolemia and normal circulating blood volume to prevent DCI (Class I; Level of Evidence B). Reference Ferguson and Macdonald1 Hypervolemia, antiplatelet therapy, and balloon angioplasty are not recommended (Class III, Level of Evidence B) as DCI prophylaxis. Reference Connolly, Rabinstein and Carhuapoma3,Reference Zwienenberg-Lee, Hartman and Rudisill23,Reference Dorhout Mees, van den Bergh, Algra and Rinkel24 Despite a lack of recommendations supporting its use, a minority of respondents in this survey indicated they would use hypervolemia as DCI prophylaxis.
Monitoring for DCI
Early detection of DCI is difficult, particularly in poor grade aSAH patients who do not consistently manifest symptoms and in whom clinical examination is limited; they unfortunately represent the most at-risk group. Reference Francoeur and Mayer8 The majority of respondents indicated that patients should be monitored in the ICU for > 7 days following aSAH; this was expected as DCI and angiographic vasospasm are common following aSAH, often occurring 3–12 days after rupture. Reference Weir, Grace, Hansen and Rothberg25 The use of CTA between day 4 and 8 post-aSAH is recommended by some as a first-line modality. Reference Francoeur and Mayer8 Interestingly, 18.3% of study respondents utilize this method.
The majority of study respondents (67.8%) indicated use of noninvasive TCD ultrasonography as a screening modality for DCI. This is in keeping with AHA/ASA Guidelines for Management of aSAH (Class IIa; Level of Evidence B), which recommend the use of TCDs to monitor the development of arterial vasospasm. Reference Connolly, Rabinstein and Carhuapoma3 A downside of TCD ultrasonography as a screening tool for DCI, as with vascular imaging, is that vessel spasm detected by TCDs does not directly translate to a high risk of DCI. Reference Carrera, Schmidt and Oddo26 Screening is complicated as many patients with large artery spasm never develop symptomatic ischemia, whereas others with modest spasm develop symptoms and even radiographic infarction. Reference Vergouwen, Vermeulen and van Gijn27 Lastly, TCD is operator-dependent, which may partially explain why a substantial proportion of respondents (18.3%) indicated that they obtain a CTA on day 7 post-aSAH, either alone, or as an adjunct to TCDs, screening for DCI.
Monitoring for the consequences of large and small vessel vasospasm by means of perfusion CT and MR imaging represents a useful adjunct, as opposed to, or combined with assessment of vessel narrowing. Reference Dankbaar, de Rooij, Velthuis, Frijns, Rinkel and van der Schaaf28,Reference van der Schaaf, Wermer, van der Graaf, Hoff, Rinkel and Velthuis29 Indeed, AHA/ASA aSAH guidelines suggest perfusion imaging with CT or MRI can be useful to identify regions of potential brain ischemia (Class 2a; Level of Evidence B). Reference Connolly, Rabinstein and Carhuapoma3 Although CT perfusion correlates well with DCI, a downside of this modality is the degree of variability limiting generalizability in absolute threshold values; this owes to differences in equipment and postprocessing. Reference Sanelli, Ugorec and Johnson30 However, this technique is often utilized in addition to CTA and serial TCDs. Notably, no randomized trials have compared the efficacy of various diagnostic methods with respect to patient outcomes.
Initial Treatment of DCI
Although DCI has been defined by expert consensus, Reference Vergouwen, Vermeulen and van Gijn27 the inconsistency in the use of this definition makes comparison of treatment efficacy across clinical trials difficult. The results of this survey demonstrate a lack of consensus among respondents regarding the indications (e.g., clinical deficits, radiographic vasospasm, or failed prophylaxis) for initiating hyperdynamic therapy. This lack of consensus may reflect a lack of standardized definition of DCI across and within institutions. Hyperdynamic therapy by means of inducing arterial hypertension is recommended for patients with DCI unless blood pressure is elevated at baseline or cardiac status precludes it (Class 1; Level of Evidence B). Reference Connolly, Rabinstein and Carhuapoma3 We identified significant variability in first-line management of DCI; this was the case for both centers with and without standardized treatment protocols. Consensus was lacking among respondents regarding the ideal milrinone starting dose. Despite the existence of AHA/ASA Guidelines for aSAH and DCI, the majority of respondents indicated “none” regarding evidence guiding management.
Salvage Therapies for DCI
AHA/ASA aSAH guidelines recommend cerebral angioplasty (either physical or chemical) as reasonable options for patients with DCI, particularly in patients who are not responding to hyperdynamic therapy (Class 2a; Level of Evidence B). Reference Connolly, Rabinstein and Carhuapoma3 This survey found IA therapy was recommended as first-line therapy by a small minority of respondents, whereas it was the most recommended salvage therapy (75%). A minority of participants felt that >75% of patients with DCI will require a form (i.e., chemical or physical) of IA angioplasty (13% chemical and 4% physical). In a recent study of patients receiving IV milrinone for cerebral vasospasm with DCI, 76% experienced significant improvement in their neurological status within 24 hours of initiating IA milrinone. Moderate/severe radiological vasospasm independently predicted the need for rescue therapy (OR 27, 95% CI 8.01–112). Reference Abulhasan, Jimenez, Teitelbaum, Simoneau and Angle15
Only 12.5% of respondents indicated milrinone as their second-line management of DCI. The starting dose of milrinone varied. Most respondents indicated that they started with a higher starting dose of 0.5 mg/kg/min. There are no evidence-based guidelines to direct the initiation of milrinone therapy. Of respondents, 14.3% used a bolus dose either to augment an infusion or alone, and one respondent used intra-arterial super-selective milrinone. Most respondents that reported using milrinone (78.5%) chose a starting dose between 0.25 and 0.5mg/kg/min with no bolus.
Next Steps
Given the well-described association between DCI and unfavorable outcome after aSAH, Reference Rosengart, Schultheiss, Tolentino and Macdonald2 the variability in treatment patterns presents a potential avenue for improving care for this patient population. Interestingly, a substantial proportion of respondents reported using milrinone as a salvage therapy for DCI, despite the paucity of evidence for its use, highlighting the need for more work to be undertaken in this area. We anticipate the results of this survey may be used toward the development of national guidelines for management of patients with DCI.
Limitations
Limitations of this study relate to its cross-sectional survey design. We assessed clinician preference, which presents the potential for selection bias in that individuals with an interest in DCI would be more likely to complete the survey. In addition, free-text fields were not uniformly answered by all respondents, leading to a heterogeneity in completeness of these fields. As such, many of the more detailed, qualitative parts of the data were not wholly reliable, with variation between differentially answered fields. However, current DCI management guidelines are limited regarding evidence quality, thus warranting this further investigation to describe national practice patterns.
Conclusion
We identified several areas of agreement regarding the management of DCI. These included the need for monitoring in an ICU via clinical and radiographic modalities, as well as the use of prophylaxis for the prevention of DCI. We also identified variability across responses regarding the indication for starting hyperdynamic therapy, and the proportion of clinicians that reported using IV milrinone, IA vasodilators, or physical angioplasty for the treatment of DCI. Furthermore, our findings highlight deficiencies regarding the use of standardized definitions, and protocols for the work-up and management of DCI. These results may be used toward the development of national standards in the definition and management of DCI.
Conflicts of Interest
The authors have no conflicts of interest to declare. The CNRC has no conflicts of interest, including affiliations with, or involvement in, any organization or entity with any financial interest, or non-financial interest in the subject matter or materials related to the study. This study was conducted with the support of the Canadian Neurosurgical Society and the Canadian Neurocritical Care Society.
Statement of Authorship
ME, MKS, RB, CIM, and JT were involved in study conceptualization and design. ME, MM, MKS, TD, AP, AA, RB, CIM, LE, MR, SP, SC, and JT were all involved in data collection, analysis, manuscript preparation, and review. This study was conducted through the Canadian Neurosurgery Research Collaborative and would like to acknowledge its members for their support in the research process.
Appendix 1
American Heart Association/American Stroke Association Guidelines for the Management of Aneurysmal Subarachnoid Hemorrhage (2012) as related to the diagnosis and management of DCI after aSAH. Reference Connolly, Rabinstein and Carhuapoma3
-
1. All patients should receive prophylactic nimodipine as it has been associated with improved neurological outcomes after aSAH, though not a decrease in radiographic vasospasm (Class 1; Level of Evidence A).
-
2. Clinicians should target euvolemia and a normal circulating blood volume to prevent DCI (Class 1; Level of Evidence B).
-
3. It is not recommended to use prophylactic hypervolemia or balloon angioplasty in the absence of radiographic vasospasm (Class 3; Level of Evidence B).
-
4. It is reasonable to use transcranial Doppler to monitor for the development of vasospasm (Class 2a; Level of Evidence B).
-
5. CT or MR perfusion imaging may be useful to identify potential regions of brain ischemia (Class 2a; Level of Evidence B).
-
6. For patients with DCI, induced hypertension is recommended unless blood pressure is elevated at baseline, or they have cardiac contraindications (Class 1; Level of Evidence B).
-
7. Rescue therapy with cerebral angioplasty or intra-arterial vasodilator therapy is reasonable for patients with DCI that do not rapidly improve with induced hypertension (Class 2a; Level of Evidence B).







