Autonomic changes including cardiac (tachy and bradyarrhythmias, hypertension), respiratory (“neurogenic” pulmonary edema), and cutaneous (piloerection, sweating) are known manifestations of temporal lobe seizures.Reference Baumgartner, Lurger and Leutmezer1 Interestingly, insular seizures can also manifest with cardiac changes, in addition to abnormal somatosensory or vicerosensory experiences.Reference Baumgartner, Lurger and Leutmezer1–Reference Craig, Chen, Bandy and Reiman3 The pathophysiology underlying these changes involves disruption of the central autonomic network (CAN). This network is comprised of structures in the insular, temporal (amygdala and stria terminalis), and frontal (prefrontal cortex) lobes, as well as in deep (hypothalamus) and brainstem structures.Reference Baumgartner, Lurger and Leutmezer1, Reference Benarroch4, Reference Mraovitch and Calando5 The CAN is an essential controller of primitive bodily functions including maintenance of homeostasis and visceral functions.Reference Benarroch4 Patients presenting with dysautonomia and its downstream effects have a wide differential diagnosis, but focal seizures with autonomic features should be considered. We report a case of autonomic dysfunction associated with non-dominant temporal lobe seizures, likely with propagation from the insular cortex, in an 83-year-old female.
An 83-year-old right-handed woman with medical history significant for right hemispheric infarct secondary to carotid atherosclerosis, and remote follicular lymphoma in sustained remission after treatment with bendamustine and rituximab, presented with acute encephalopathy. This culminated in a witnessed generalized seizure, with laboratory evidence of multi-organ failure at presentation to hospital. She and her family members described a normal morning and afternoon with nausea, dizziness, and dysgeusia developing suddenly in the evening. These symptoms fluctuated over multiple hours, evolving to include hyperventilation, anxiety, and diaphoresis with urinary urgency. Family members describe fluent speech and complaints of "blurry vision” and unsteadiness. Emergency medical services were contacted and found the patient to be afebrile, tachycardic, tachypneic, and hypertensive (blood pressure 220/120 mmHg). She was transferred to the regional hospital and en-route had a 1–2 minute episode of limb stiffening and shaking with altered level of consciousness.
On arrival to hospital, there was persistent hypertension (246/121 mmHg), tachycardia, and tachypnea, and the patient was drowsy but alerted slightly to tactile stimulation. A non-contrast computed tomography (CT) scan of the head demonstrated right temporal-insular-parietal encephalomalacia consistent with the reported prior stroke (Figure 1A). She had a markedly elevated serum troponin, diffuse ST depression on electrocardiogram (ECG), acute kidney injury with hematuria and proteinuria, pulmonary edema (Figure 1B), and metabolic acidosis. After exclusion of aortic dissection, the patient’s hemodynamics were stabilized with diuresis and nitroglycerin infusion. This resulted in improvement in her level of consciousness within hours to near baseline, with some persistent slowing of verbal responses and retrograde amnesia for the preceding 24 hours. Despite overall improvement in awareness, in follow-up, the patient described having ongoing episodes of sensory experiences lasting seconds to minutes alternating between “shaved ice” and warmth descending from her pharynx to abdomen. It came to light that these sensory spells had been occurring less frequently for days to weeks prior to presentation, at times accompanied by negative thoughts.
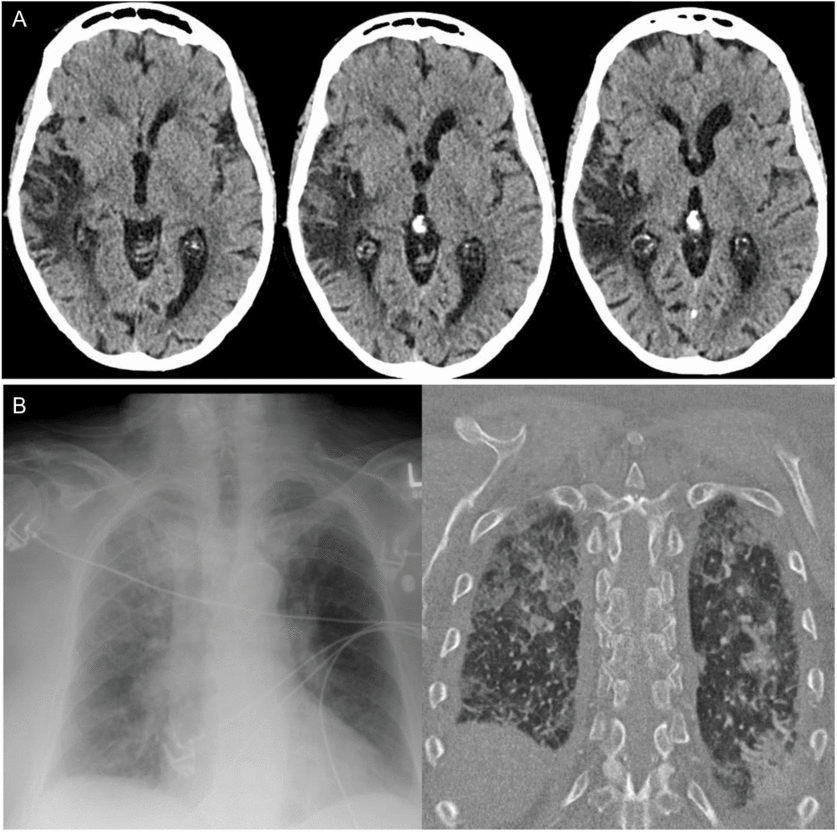
Figure 1: Head and chest imaging at presentation. (A) Axial CT non-contrast head slices revealing previous infarct involving the superior temporal, posterior insular, and parietal lobes and (B) Chest x-ray and CT findings of bilateral alveolar infiltrates consistent with pulmonary edema.
Posterior reversible encephalopathy syndrome (PRES) was considered a diagnostic possibility given the combination of encephalopathy, hypertension, and renal dysfunction in the setting of immunosuppression. PRES usually occurs in patients with endothelial dysfunction and labile blood pressure and can present to neurological care with encephalopathy, focal seizures, vision loss, or headache. Our patient was considered at risk given prior exposure to rituximab (which has been reported in association with PRES although less commonly than with tacrolimus or cyclosporine).Reference Zito, Lee, Johnson, Singer and Vacirca6
Other potential causes for focal seizures in this patient included leptomeningeal or parenchymal recurrence of follicular lymphoma, ischemic stroke, or encephalitis (though we were reassured by the absence of infectious signs and clinical improvement without empiric treatment for encephalitis). A paraneoplastic process was considered unlikely given the acuity of onset.
Magnetic resonance imaging (MRI) of the brain (Figure 2A) unexpectedly showed isolated diffusion restriction in the right mesial temporal lobe and electroencephalogram (EEG) revealed right anterior and mid-temporal epileptiform discharges (Figure 2B). The post-test probability of a viral encephalitis had increased, so although the patient remained afebrile and neurologically unchanged; intravenous acyclovir and antiepileptic drug therapy with levetiracetam were initiated, and a lumbar puncture was performed. This showed normal acellular cerebrospinal fluid with both negative cultures and viral polymerase chain reaction (including herpes simplex). Antiviral therapy was discontinued, and the patient remained hemodynamically and neurologically stable on levetiracetam. A thorough work-up for secondary causes of hypertension was pursued in hospital with imaging and laboratory tests excluding endocrinopathy, renal artery stenosis, and pheochromocytoma. The patient was discharged home at her neurologic baseline with a new diagnosis of epilepsy.
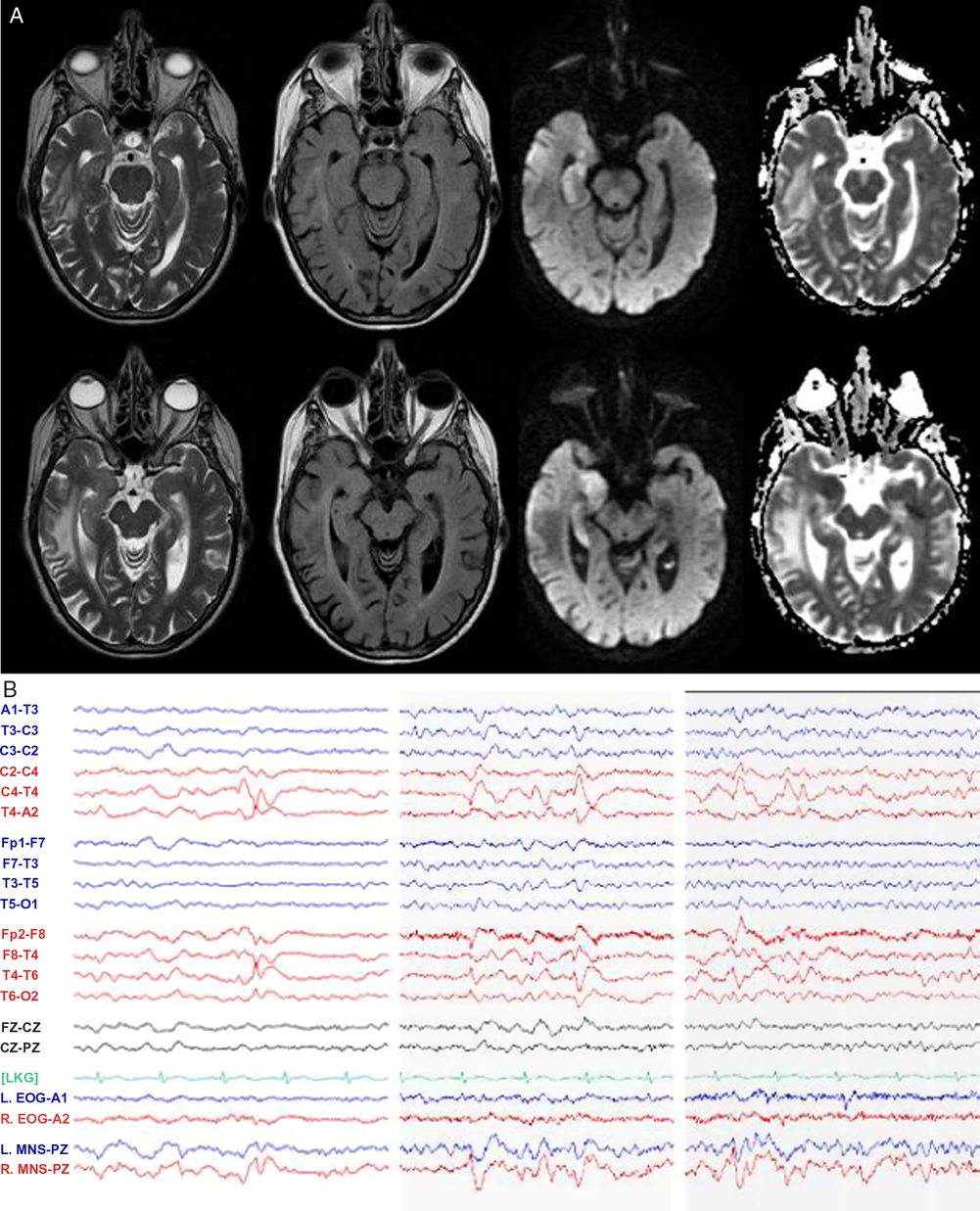
Figure 2: MRI and EEG day 2 after presentation. (A) MRI T2-weighted, T2 fluid attenuated inversion recovery (FLAIR), diffusion-weighted imaging (DWI), and apparent diffusion coefficient (ADC) sequences demonstrating right mesial temporal lobe restricted diffusion and (B) EEG demonstrating right anterior and mid-temporal epileptiform discharges at T4 and F8-T4. n.b MNS “mandibular notch surface.”
We report a case highlighting the significant autonomic changes that can accompany temporal lobe seizures, through disruption of the CAN.Reference Baumgartner, Lurger and Leutmezer1, Reference Benarroch4, Reference Mraovitch and Calando5 Clinical studies suggest that these functions are lateralized with right hemispheric ictal activity accompanied by sympathetic excess (e.g. tachyarrhythmia, hypertension), whereas ictal bradyarrhythmia may be more common with left temporal seizures.Reference Baumgartner, Lurger and Leutmezer1, Reference Galli and Lombardi7–Reference Chouchou, Bouet, Pichot, Catenoix, Mauguiere and Jung9 An analogy and memory aid for the right hemisphere “go” and left hemisphere “slow” signal is the configuration of the gas pedal and brake pedal in a car. Additional features, as were present in our case include hypertension and “neurogenic pulmonary edema,” thought to be driven by sympathetic nervous system overactivation in acute central nervous system dysfunction.Reference Devinsky2 Although impossible to definitively rule out other causes of pulmonary edema, we suggest a neurogenic cause given the findings of acute dyspnea, tachypnea, and tachycardia in our patient, accompanied by chest x-ray imaging revealing alveolar opacities with a normal-sized heart, as well as echocardiogram revealing normal systolic ejection fraction. On review by cardiology, the ST segment changes on ECG were considered related to severe hypertension superimposed on coronary artery disease; however, ECG abnormalities can result from insular seizures; as well, cardiac type chest pain has been previously described in seizures involving limbic structures.Reference Baumgartner, Lurger and Leutmezer1, Reference Benarroch4 Urinary urge, as in our case, has previously been described in non-dominant temporal lobe seizures, with single photon emission computed tomography (SPECT) imaging highlighting hyperperfusion of the insula and superior temporal gyrus.Reference Devinsky2, Reference Baumgartner, Gröppel and Leutmezer10 The recurrent viscerosensory spells linked to negative thoughts, revealed in follow-up, coupled with the evidence of encephalomalacia of the posterior insula on CT scan (Figure 1A), suggest that our patient’s focal seizures may have started in the insula with propagation to medial temporal structures, resulting in dysautonomia and the restricted diffusion present on MRI (Figure 2).
Non-dominant, focal temporal, and insular seizures can be subtle, particularly if aphasia and focal motor activity are absent. The spectrum of clinical presentation is broad and can involve sympathetic overactivity as is highlighted in the current case, as well as a range of uncommon ictal phenomena including panic and anxiety symptoms.Reference Sazgar, Carlen and Wennberg11 Here, the formulation of PRES as a secondary feature of hypertension was overturned on the basis of ancillary testing and inference, providing an example of focal seizures causing severe dysautonomia and its downstream effects.
Funding
No targeted funding reported.
Statement of Authorship
Both AP and DW contributed to study concept, authorship, and editing.
Disclosures
Both AP and DW report no disclosures.