Huntington’s disease (HD) is an autosomal dominant neurodegenerative disorder characterized by the triad of progressive chorea, cognitive decline and psychiatric symptoms. Spontaneous intracranial hypotension (SIH) has a varied clinical presentation and may include a movement disorder.Reference Salazar 1 , Reference Pakiam, Lee and Lang 2 We report a rare case of exacerbation of HD due to SIH.
This 62-year-old right-handed gentleman presented in 2008 with facial chorea and was genetically proven to have HD. His symptoms remained stable for several years. He required no treatment. He was fully independent and working full time as a nuclear engineer. In 2016, he developed a progressive holocephalic orthostatic headache associated with marked fatigue, nausea and vomiting, and gait disturbance. After several months he developed worsening and more widespread chorea such that he was unable to work and required assistance with his activities of daily living. He also had progressive cognitive decline (5-point decrement on repeat MoCA 23/30). Brain MRI revealed findings suggestive of SIH with downward sagging of the brain, diffuse pachymeningeal enhancement (Figure 1A) and bilateral small subdural hygromas (SDHs) (Figure 1B). Spinal CT myelography identified a possible small CSF leak posteriorly at the C1-C2 level. This was treated twice with CT-guided epidural blood patch (EBP) with only temporary improvement. A week later he developed worsening chorea, disorientation and hallucinations. It was thought his clinical change was consistent with Huntington’s and he was started on zopiclone and risperidone.
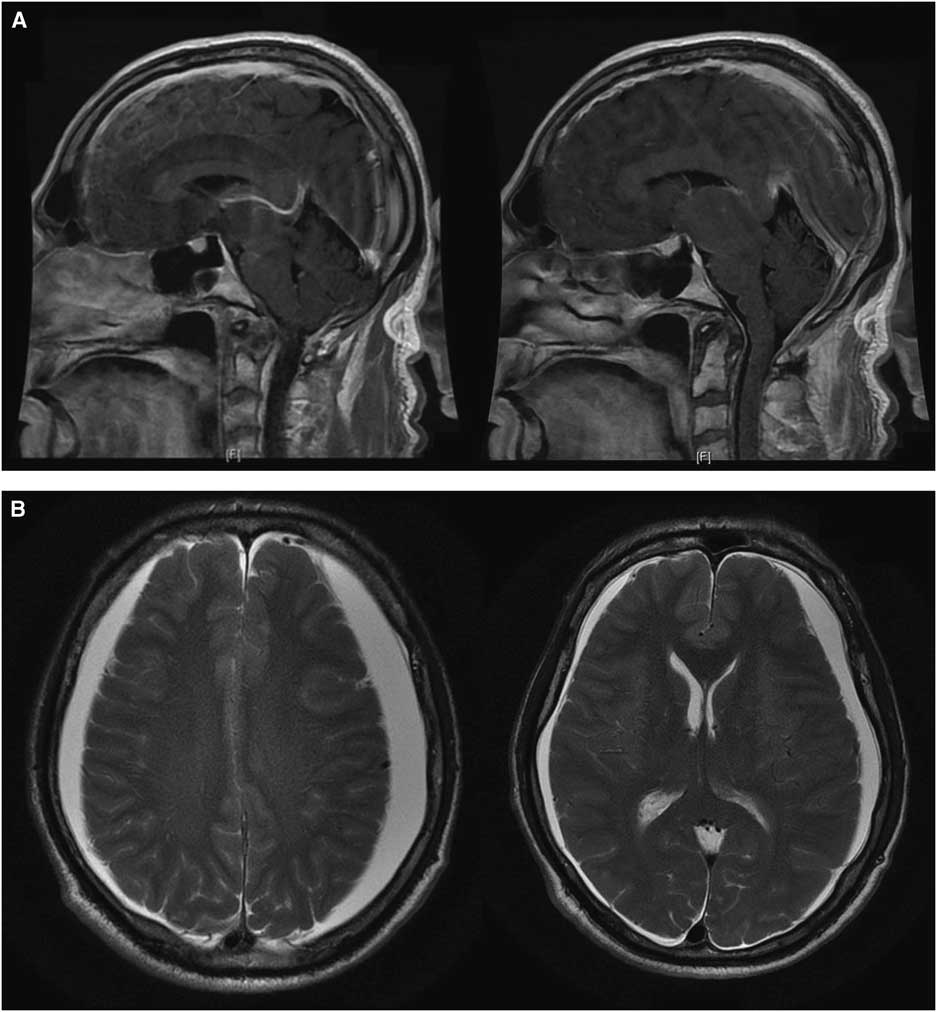
Figure 1 (A) Initial T1 sagittal post-gadolinium brain MRI slices demonstrating radiologic findings of spontaneous intracranial hypotension including diffuse pachymeningeal enhancement, downward displacement of the brain with sagging of the brainstem, and effacement of suprasellar and prepontine cisterns. (B) Initial T2 axial brain MRI slices showing enlargement of subdural spaces with CSF signal compressing both cerebral hemispheres with minimal left-to-right midline shift.
However, repeat CT head suggested worsening SIH with an enlarged left SDH resulting in mild midline shift (Figure 2). A large-volume EBP was performed from spinal levels T2 through S1 to a total volume of 56 ml.Reference Staudt, Pasternak and Sharma 3 After a week, he was free of headache, oriented in all spheres, and had no further hallucinations. However, with the increased left SDH and left-to-right shift, burr hole evacuation was also performed. Upon discharge from the hospital, he remained oriented, with no headache, no psychiatric symptoms, significantly improved cognition (MoCA 29/30) and stable gait. At 3-month follow-up, the patient was headache-free, his chorea was at baseline, he was driving and had resumed work. MoCA was 29/30. MRI showed small bilateral SDHs with improved configuration of the midline brain structures and resolution of brain slumping (Figure 3).
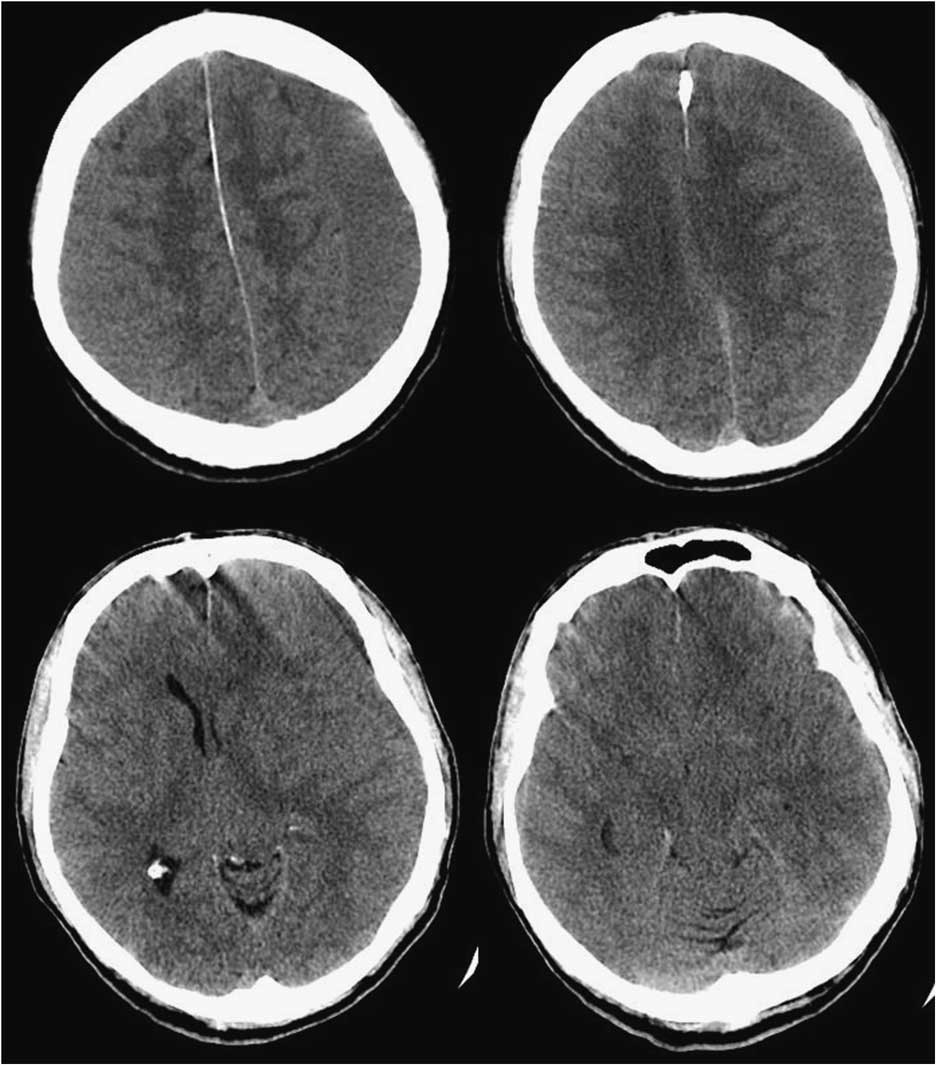
Figure 2 Axial brain CT slices demonstrating enlarged left subdural hygroma with significant left-to-right midline shift.
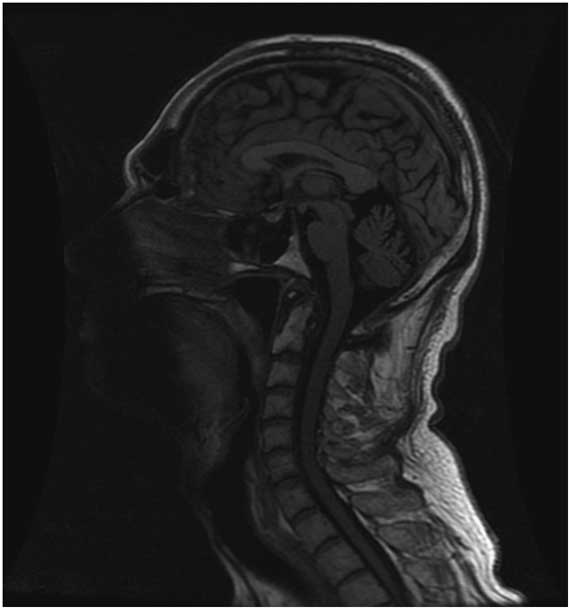
Figure 3 T1 sagittal brain MRI at 3-month follow-up showing radiologic improvement of spontaneous intracranial hypotension including improvement of the configuration of the brain midline structures, decreased downward displacement of the brainstem and less supratentorial parenchymal slumping.
We report a case of transient worsening of HD chorea and cognition associated with orthostatic headaches and proven SIH. The clinical deterioration observed was initially erroneously attributed to progression of HD. Given the exceptional improvement achieved after large-volume EBP and evacuation of the chronic SDH, the case highlights that the symptoms were without question secondary to the SIH.
The initial sign of SIH was the orthostatic headache, likely due to traction.Reference Pakiam, Lee and Lang 2 , Reference Mokri 4 The progression to “non-headache manifestations” (e.g., Parkinsonism, ataxia, bulbar symptoms, frontotemporal dementia, gait disorder, behavioral disorder and chorea) is consistent with previous reports. MokriReference Mokri, Ahlskog and Luetmer 5 presented a series of five cases of new onset movement disorder associated with spontaneous CSF leak and imaging findings of SIH. Thus, SIH may mimic a seeming progression of HD.
A second contributing factor in the case may have been the SDH causing mild midline shift. HD patients are at higher risk for SDH due to cortical atrophy.Reference Sipilä, Posti and Majamaa 6 , Reference Pechlivanis, Andrich, Scholz, Harders, Saft and Schmieder 7 Reports exist of movement disorders developing secondary to chronic SDH. Gilrnore and BrennerReference Gilrnore and Brenner 8 speculated that the development of chorea may be secondary to tentorial herniation and damage to deep structures. Bae et al.Reference Bae, Vates and Kenton 9 describe two patients with bilateral chronic SDH presenting with symmetric generalized chorea that improved or resolved after evacuation. The authors thought that symptoms were secondary to ischemia of the striatum caused by mass effect. In our case, the patient’s clinical picture had already improved substantially after the large-volume EBP. The SDH was evacuated because small foci of more acute SDH were observed on CT and MRI. At surgery, the SDH was under mild-to-moderate pressure. Had the brain failed to respond, an intraoperative spinal saline infusion could have been considered to assist re-expansion.Reference Stephen, Rojas, Lioutas, Papavassiliou and Simon 10
This case suggests that unrelated conditions such as SIH need to be considered in individuals presenting with exacerbation of a pre-existing movement disorder. More importantly, symptoms may be reversible with treatment of SIH.
Disclosure
ELF, MSJ, DMP, and SPL have nothing to disclose.
Statement of Authorship
ELF and SPL: Conception and design; and analysis and interpretation of data.
ELF, DMP, SPL: Acquisition of data.
ELF, MSJ, DMP, SPL: Drafted the article; critically revised the article; reviewed submitted version of manuscript.
ELF: Approved the final version of the manuscript on behalf of all authors.





