Background/Objective
Ischemic stroke is a rare but serious complication of pregnancy, affecting approximately 12/100,000. Reference Swartz, Cayley and Foley1 Case fatality rate of pregnancy-associated ischemic stroke in a recent Canadian study was 7%. Reference Liu, Chan, Ray, Kramer and Joseph2
Although intravenous tissue plasminogen activator (IV-tPA) and endovascular thrombectomy (EVT) are effective for treatment of acute ischemic stroke, Reference Emberson, Lees and Lyden3,Reference Goyal, Menon and van Zwam4 clinical trials examining these therapies, unsurprisingly, excluded pregnant women. There are no large, randomized studies investigating the safety or efficacy of these treatment options in pregnancy or in the post-partum period. The published experience with respect to acute stroke therapy in pregnant women is limited. In these published cases, IV-tPA was administered during all trimesters and, in general, most mothers made a good recovery and delivered healthy babies with incidence of symptomatic intracerebral hemorrhage being comparable to that in non-pregnant patients. Reference Sousa Gomes, Guimarães and Montenegro5,Reference Demchuk6 The published experience with endovascular therapy in pregnancy is also limited; most cases reported are in the third trimester of pregnancy and with good outcomes for both the mother and baby. Reference Limaye, Van de Walle Jones and Shaban7
The experience of treating acute ischemic stroke in pregnancy is not well documented, and preferred patterns of practice in Canada are not known. In 2018, recognizing a need for clinical guidance, the Canadian Stroke Best Practice and Quality Advisory Committee released a joint consensus statement for treating acute stroke in pregnancy. Reference Ladhani, Swartz and Foley8 We sought to describe real-life patterns of practice amongst stroke specialists with regards to IV-tPA and EVT in pregnant and post-partum patients.
Methods
The study was approved by the Clinical Research Ethics Board at the University of British Columbia. Participants were recruited through the Canadian Stroke Consortium (CSC) email list, which had 111 physician members involved in treatment of stroke, mostly neurologists. The survey consisted of seven main questions with branching sub-questions regarding past experience and theoretical treatment preferences with respect to reperfusion therapy for acute ischemic stroke in pregnant and post-partum women and demographic information. We defined the immediate post-partum period as <1 week and late post-partum period as ≥1 week. The survey was available to respondents in either English or French (see Supplement). Responses were de-identified. An unconditional $5 coffee gift card was offered to all participants. The survey remained open between October 2018 and April 2019, and one reminder email was sent 6 weeks after the initial invite.
Individual participant responses were exported from the survey’s web interface (Qualtrics University platform) into SPSS 26 (Armonk, NY) and analyzed using descriptive statistics, Chi-square, and Wilcoxon rank-sum tests as applicable. A p-value of <0.05 was considered significant. No formal adjustments were made for multiple comparisons but results were interpreted in light of this context. Missing data was non-random and thus treated with pair-wise deletion. Free-text responses were examined manually and coded for thematic content using an inductive data-driven approach. Reference Braun and Clarke9 One author (TSF) coded the data with similar codes grouped to form sub-themes that were then reviewed for agreement by a second author (AB). Sub-themes were then integrated to identify main themes. Disagreements were resolved by consensus.
Results
Thirty-six of 111 (32%) physician members of the CSC mailing list participated with representation from most provinces. One respondent registered for the unconditional incentive but did not complete the survey; data from 35 participants are thus reported. One-third of respondents identified as female. The vast majority of respondents were stroke specialists and over half of respondents dedicated >75% of their clinical time to cerebrovascular disease. Career stages were evenly distributed (Table 1).
Table 1: Demographic characteristics of respondents (n = 35)

Ten participants (29%) had given IV-tPA to a pregnant woman at least once; two had experience with two cases. Most instances were in the third trimester. Two additional participants had cases where the patient was otherwise IV-tPA-eligible but thrombolysis was not administered because the patient would not give informed consent (n = 1) or because the respondent had not felt comfortable administering IV-tPA to a pregnant patient (n = 1) (Table 2). Of those who had experience with tPA in pregnancy, none reported any treatment-associated complications. There were no associations or trends with respect to respondent demographics (gender, years in practice, practice time dedicated to stroke) and experience with administration of IV-tPA in pregnancy.
Table 2: Treatment experience and preferences regarding reperfusion therapy in pregnant patients
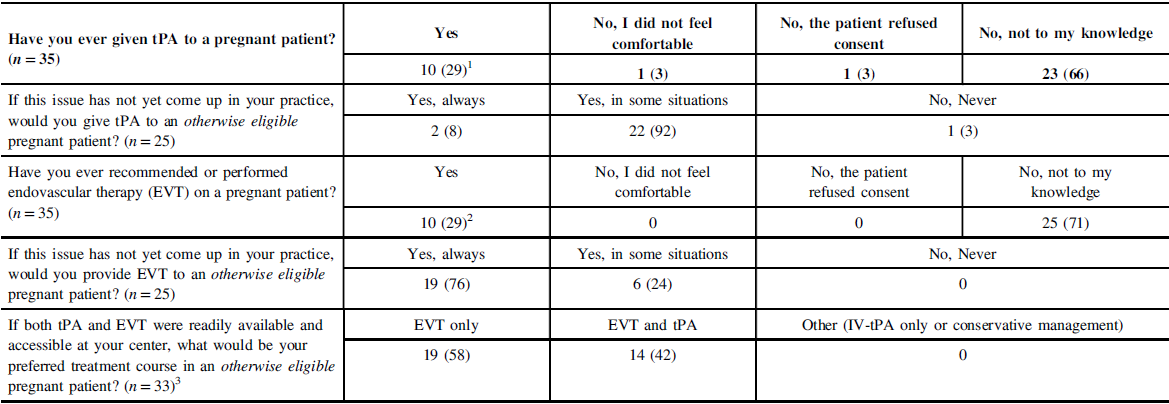
1 First trimester (n = 2); second (1), third (5); responders with two patients (n = 2): second and third (1), can’t recall (1).
2 First trimester (n = 2), second (2), third (6).
3 Two non-respondents for the question.
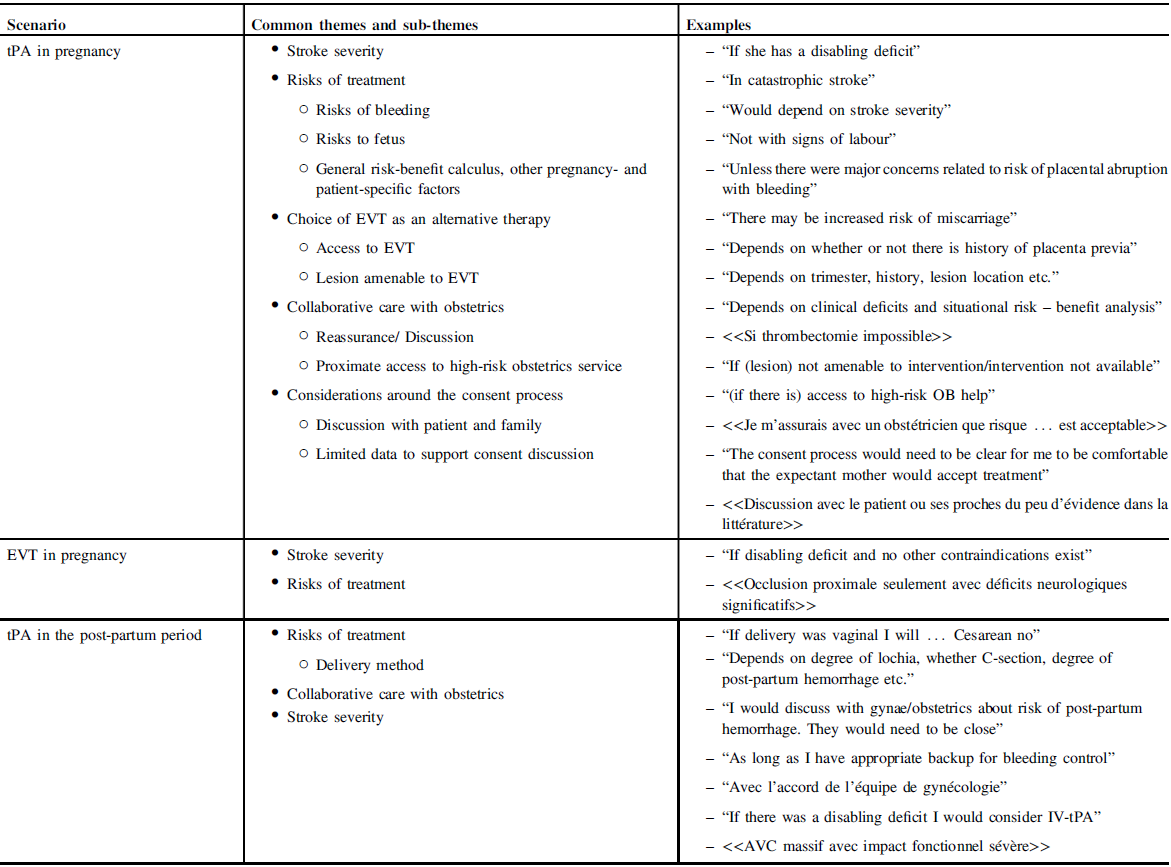
Respondents who had never encountered the scenario were asked if they would proceed with IV thrombolysis in the case of an otherwise IV-tPA-eligible pregnant patient. Only two respondents (8%) answered with an unqualified “yes, always”; one with “no,” and the remaining 22 (88%) with qualifications (“yes, in some instances”) (Table 2). Themes arising in the qualifications discussed by respondents included (a) stroke severity, (b) risks of treatment and/or risk-benefit calculus, (c) alternative therapies, (d) collaborative care with obstetrics, and (d) considerations around the consent process (Table 3).
Table 3: Thematic analysis and examples of responses qualifying “yes, in some situations” responses
Ten participants (29%) had experience with EVT in pregnancy, mostly during the third trimester. Of those, seven had also given IV-tPA to pregnant patients, though there was no mechanism to clarify if both treatments were administered to the same patient. Two respondents had experience with EVT but not tPA. There were no instances where an EVT-eligible patient did not proceed with therapy due to issues with consent or physician comfort. None experienced EVT-related complications, although one respondent’s patient required an emergency induction following EVT due to atypical HELLP syndrome as well as concerns requiring the need for possible hemicraniectomy (Table 2). There were no associations between respondent demographics and experience with EVT in pregnancy.
Respondents who had never encountered the scenario were asked if they would proceed with endovascular therapy in the case of an otherwise EVT-eligible pregnant patient. Nineteen (76%) respondents gave an unqualified “yes, always” with the remaining six answering “yes, in some instances” (Table 2). EVT-related considerations primarily focused on stroke severity and risk-benefit calculus, with a lesser focus on the consent process (Table 3).
All respondents were asked as to how they would proceed in the theoretical case of an otherwise IV-tPA- and EVT-eligible pregnant patient, where both therapies were readily available. Fourteen (40%) responded that they would proceed with both IV-tPA and EVT and 19 (54%) with EVT only, with 2 non-responses. Three of those who responded with “EVT-only” had experience giving IV-tPA in pregnancy. There was no association between treatment preference and respondents’ demographic characteristics.
Two respondents had experience giving IV-tPA to women in the early post-partum period. One case was complicated by major hemorrhage requiring transfusion or surgical intervention. Seven respondents (20%) had given IV-tPA to women in the later post-partum period (ranging from 2 weeks to 3 months), none of whom had any complications (Supplementary Table S1). Those who had not experienced these scenarios for the most part expressed hesitancy in giving IV-tPA in the early post-partum period. Considerations focused mainly on bleeding risks and in particular as they related to delivery method. Collaborative care with obstetrics was emphasized, with several participants focusing on urgent physical availability of the obstetrics–gynecology team (Table 3). More participants were amenable to administration of IV-tPA in the later post-partum period though the reported considerations were similar.
Discussion
Amongst respondents, experience with reperfusion therapy in pregnancy was not uncommon. Over one-third (12/35) of respondents had experienced cases involving use of IV-tPA, EVT or both in pregnant patients. Reassuringly, no respondents reported any complications related to either therapy. Experience with thrombolysis in the early post-partum period was uncommon, with one of two cases experiencing a hemorrhage requiring treatment. Most respondents who had not yet encountered such a scenario in practice showed an overall willingness to recommend EVT for eligible cases in pregnancy, but a large proportion expressed hesitancy towards using IV-tPA in pregnancy, particularly in a situation where the patient was also EVT eligible.
The Canadian Best Practice Consensus statement on acute stroke management in pregnancy recommends prioritizing maternal health in clinical decision-making. It also recommends employing, whenever possible, the same clinical decision-making process that one would make for a non-pregnant patient. Reference Ladhani, Swartz and Foley8 In the context of limited literature regarding safety of IV-tPA and EVT in pregnancy, the fact that no complications with either treatment are reported here should provide additional reassurance of safety for reperfusion therapies in properly selected pregnant patients. It should be noted, however, that our respondents for the most part represent a specialized subset of stroke specialists at comprehensive stroke centers.
It is unclear whether the apparent hesitancy to use IV-tPA in pregnancy reflects clinician risk aversion specific to pregnant patients. The recurrent themes in respondent comments suggest this is the case given frequent mention of the need for shared decision-making with obstetrics and express consent from the patient and family. Alternatively, the lack of clinician enthusiasm for IV-tPA may be more indicative of equipoise regarding use of IV thrombolysis in EVT-eligible patients in general. Reference Fischer, Kaesmacher and Mendes Pereira10 The latter question continues to be explored in ongoing clinical trials. At present, the available evidence does not suggest the benefit of withholding IV thrombolysis in otherwise eligible patients. Reference Vidale, Romoli, Consoli and Agostoni11 Interestingly, respondents were hesitant but few were opposed to recommending IV-tPA in the early post-partum period, where the reported experience suggests that risk of hemorrhage may be high. In a case series of 13 patients administered IV thrombolysis for pulmonary embolism (n = 12) and acute stroke (n = 1) in the very early post-partum period (<48 h post-partum), blood transfusions were required in all but one case. Reference Akazawa and Nishida12
Our study is limited by a response rate of one in three, though this rate is consistent with what is reported from other physician web-based surveys. Reference VanGeest, Johnson and Welch13 In addition to responder biases, our findings may also be subject to recall or reporting bias from participants. Finally, in the time since our survey was conducted, uptake of recommendations from the consensus statement may have improved.
Conclusion
In this cohort consisting mainly of stroke neurologists practicing at comprehensive stroke centers, more than a third had experience in treating acute stroke in pregnancy with tPA and/or EVT. No treatment-related complications were reported. Even in this sub-specialized group of respondents and despite consensus guidelines recommending use of thrombolysis in otherwise eligible pregnant patients, respondents were hesitant to unconditionally recommend IV-tPA, particularly when EVT was concurrently indicated and available. Future knowledge dissemination activities related to treatment of stroke in pregnancy should take into account neurologists’ concerns regarding pregnancy-specific risks of thrombolysis and a bias towards using EVT alone as a treatment strategy.
Acknowledgments
The authors thank Karen Earl and the Canadian Stroke Consortium for facilitating survey participation.
Disclosures
TSF is supported by the Vancouver Coastal Health Research Institute, the Michael Smith Foundation for Health Research and the Heart and Stroke Foundation of Canada. TSF has received in-kind study medication from Bayer Canada and an honorarium from Servier for speaker’s bureau activities. The other authors have nothing to disclose.
Statement of Authorship
CEU – Collected and analyzed data, drafted the manuscript; SGL – Translated the survey, analyzed data and critically revised the manuscript; AMB – analyzed data, critically revised the manuscript; TSF – conceived of the project, designed the survey, analyzed data and critically revised manuscript;
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/cjn.2020.207.





