Herpes simplex virus (HSV) infection is the most common acquired cause of encephalitis.Reference Alonso-Vanegas, Quintero-Lopez, Martinez-Albarran and Moreira-Holguin1 Untreated, it is associated with significant morbidity and mortality. We present a patient with epilepsy secondary to remote HSV encephalitis who experienced reactivation following epilepsy surgery.
A 21-year-old right-handed female with epilepsy was admitted for presurgical work-up. Her history was significant for encephalitis 5 years earlier in China. She had focal aware seizures with auditory hallucinations and speech comprehension difficulties that occasionally evolved to bilateral tonic–clonic convulsions. Seven habitual seizures were recorded arising from the left anterior temporal lobe. Her interictal electroencephalography (EEG) showed intermittent left hemisphere slowing maximal in the anterior to mid-temporal region and occasional left anterior temporal sharp waves. Magnetic resonance imaging (MRI) demonstrated encephalomalacia in the left temporal pole, parahippocampal gyrus, and insula as well as left hippocampal atrophy (Figure 1). Interictal single photon emission computerized tomography showed left temporal hypoperfusion. Neuropsychological testing demonstrated left hemispheric language dominance and verbal memory deficits.
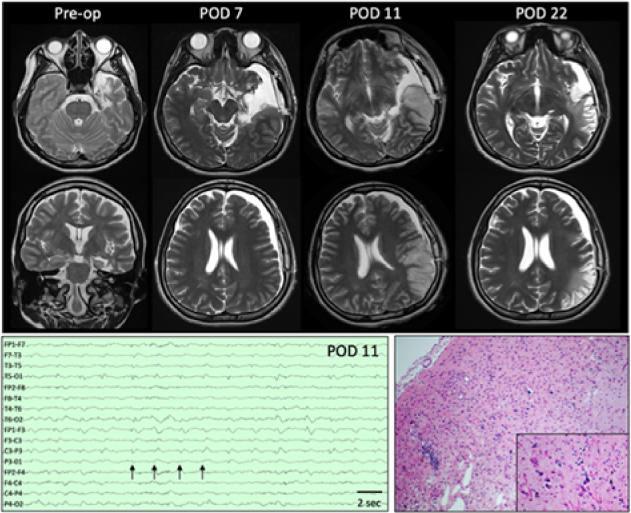
Figure 1: Top:Presurgical axial and coronal T2-weighted images demonstrate encephalomalacia in the left temporal pole, parahippocampal gyrus, and insula as well as volume loss in the left hippocampus. On POD 7, MRI showed a left subdural hygroma with mild mass effect and 5-mm midline shift as well as subtle signal change along the resection margin. Four days later (POD 11), MRI demonstrates left posterior temporal and parietal T2 hyperintensity that is predominantly cortical and accompanied by sulcal effacement. Additional sequences not shown demonstrate associated reduced diffusion and subtle leptomeningeal enhancement. By POD 11, overall decreased mass effect and swelling with developing extensive encephalomalacia are seen in the previously affected areas. Bottom left: EEG performed on POD 11 demonstrates lateralized periodic discharges maximum over the left hemisphere (arrows) as well as generalized slowing of background. Bottom right: Representative section of resected cortex showing features consistent with chronic sequelae of HSV encephalitis. Low-power view showing area of encephalomalcia with marked reactive astrogliosis, patchy cavitary tissue loss, mineralized neurons, and patchy mononuclear inflammation. Inset: High-power view highlighting the scattered mineralized neurons indicative of remote neuronal injury. Rare microglial nodules also identified (not shown). No evidence of active neuronophagia or other recent neuronal/tissue injury, viral inclusions or granulomatous inflammation to suggest active infection within the sampled tissue. (hematoxylin and eosin stain).
With a lesion and concordant electroclinical data, a left anterior temporal lobectomy was performed. Intraoperative electrocorticography showed no epileptiform discharges. Surgical temporal lobe specimens demonstrated encephalomalacia with multifocal transcortical and cortical laminar gliosis and focal cavitation. Inflammation within the parenchyma and leptomeninges was relatively sparse with occasional perivascular lymphocytic aggregates and microglial nodules. There were no viral inclusions, granulomas, or multinucleated giant cells. The pathological findings best fit with the chronic phase of herpes simplex encephalitis, including the absence of viral inclusions by histology and immunohistochemistry, but polymerase chain reaction (PCR) positivity for HSV-1.Reference Ellison, Love and Chimelli2 There were no definitive features to suggest acute activity or reactivation (granulomatous inflammation) within the resected tissue.
A dexamethasone taper (initial dose 4 mg Q6h) was started as a standard measure to reduce retraction swelling. On postoperative day (POD) 3, she had a habitual focal seizure with impaired verbal comprehension, head turn, and right-hand automatisms. Following this, her verbal comprehension and speech deteriorated. She was afebrile, and her white blood cell count was normal. Her anticonvulsants were adjusted. By POD 6, she suffered eight more seizures. EEG showed generalized slowing, and an MRI demonstrated subtle T2 hyperintensity adjacent to the resection (Figure 1). Maximum temperature that day was 37.6°C and white blood cell count increased to 16.1 × 109/L (4.0–11.0 × 109/L) only to fall back down to 10.2 × 109/L by POD 9. Anticonvulsants were adjusted. Her seizures were believed to have arisen from local postoperative irritation, and her dysphasia was thought to be postictal. On POD 9, she developed global dysphasia despite no obvious seizures. Continuous EEG was initiated. Three clinical and six electrographic seizures were recorded. Anticonvulsants were increased. She subsequently developed a fever of 38.4°C that increased to 40.2°C on POD 10. On POD 11, EEG showed left lateralized periodic discharges (Figure 1). A repeat MRI showed evolving T2 hyperintensity and restricted diffusion in the left posterior temporal and parietal lobes with regional leptomeningeal enhancement concerning for infectious encephalitis (Figure 1). The results of a lumbar puncture demonstrated 2.42 g/L protein (0.15–0.45 g/L), 2.6 mmol/L glucose (2.2–3.9 mmol/L), 17 × 106/L white blood cells with 67% monocytes (0–5 × 106/L), 59 × 109/L red blood cells, and xanthochromia. Empiric meningitic-dosed ceftazidime, vancomycin, ampicillin, metronidazole, and acyclovir were started. Cerebrospinal fluid (CSF) qPCR was strongly positive for HSV-1 at a cycle threshold of 24.Reference Wong, Pabbaraju, Wong and Tellier3 An autoimmune encephalitis panel including anti-N-methyl-D-aspartate was subsequently negative. Two days later, seizures had ceased and her fever abated, but she remained globally dysphasic.
A 3-week course of intravenous acyclovir (10 mg/kg Q8) was completed followed by maintenance valacyclovir (1 g TID × 3 months, 1 g BID × 2 months, 1 g indefinitely). Prior to acyclovir discontinuation, repeat lumbar puncture demonstrated 0.36 g/L protein (0.15–0.45 g/L), 3.3 mmol/L glucose (2.2–3.9 mmol/L), 7 × 106/L white blood cells (0–5 × 106/L), 3 × 109/L red blood cells, and no xanthochromia. CSF qPCR for HSV-1 was negative. At discharge, the patient had single-word speech and comprehension for simple commands only. Two months after discharge, she had minimal further improvement.
The clinical, radiographic, and electrophysiologic deterioration following surgery (and steroid induction), concordant with CSF qPCR positivity that cleared with treatment, is indicative of HSV-1 reactivation. Indeed, recurrent HSV encephalitis has been reported following temporal lobe, pituitary, and posterior fossa procedures.Reference Alonso-Vanegas, Quintero-Lopez, Martinez-Albarran and Moreira-Holguin1, Reference Uda, Koide, Ito, Hosono, Sunaga and Morino4 In addition to HSV recurrence related to surgery, instances have been reported in the context of immunosuppression, steroids, chemotherapy, coexisting infections, and incomplete treatment.Reference Alonso-Vanegas, Quintero-Lopez, Martinez-Albarran and Moreira-Holguin1 Relapse is believed to result from reactivation of a latent infection in the cranial nerve sensory ganglia, particularly the trigeminal ganglion or through central nervous system invasion via the olfactory bulbs. Supporting this, animal models demonstrate the presence of latent HSV in the entorhinal and olfactory cortices.Reference Stroop, McKendall, Battles, Schaefer and Jones5 In our case, we hypothesize that manipulation of previously infected tissue (e.g., entorhinal cortex, trigeminal ganglion and fibers), demonstrated on pathological examination to contain HSV, in the presence of dexamethasone, led to viral reactivation.
HSV encephalitis typically presents with fever, headache, altered level of consciousness, and focal neurologic signs including seizures.Reference Rabinstein6 In the postoperative period, headache and intermittent fevers frequently occur. In our patient, early seizures of similar semiology to previously documented seizures could have been explained by postoperative irritation of an incompletely resected epileptic focus. Only when she developed persistent high fever, dysphasia, and EEG evolution of lateralized periodic discharges, was HSV infection suspected. HSV PCR is the gold standard with >90% sensitivity and specificity but can be falsely negative in the disease’s early stages.Reference Weil, Glaser, Amad and Forghani7 The typical CSF profile of HSV encephalitis is a mild to moderate lymphocytic pleocytosis, elevated protein, and normal glucose.Reference Schiffer, Corey, Bennett, Dolin and Blaser8 If there is suspicion of the diagnosis, intravenous acyclovir must be initiated immediately before the definitive PCR result is obtained.
Although a rare complication, preoperative HSV prophylaxis in previously afflicted patients should be considered. While the efficacy of HSV prophylaxis in this setting is unknown, it remains an extraordinarily well-tolerated therapy. In patients with prior HSV encephalitis that develop fever and focal neurological symptoms, a high level of suspicion for recurrent infection must be maintained.
Disclosures
AA, MDP, LEH, WH, and PF have no conflicts of interest to declare.