OnabotulinumtoxinA (BOTOX) is approved in Canada for the treatment of numerous therapeutic indications including, but not limited to, blepharospasm, strabismus, cervical dystonia (CD), focal spasticity including the treatment of upper limb spasticity associated with stroke, dynamic equinus foot deformity resulting from spasticity in pediatric cerebral palsy (CP), and primary hyperhidrosis of the axillae. 1 These conditions represent a group of disorders that result in muscle spasm, pain, and excessive sweating and may be associated with both physical and/or emotional disability that adversely impacts a patient’s quality of life (QoL).Reference Jankovic, Esquenazi, Fehlings, Freitag, Lang and Naumann 2 Over the past few decades, onabotulinumtoxinA injections have become an integral part of the therapeutic armamentarium for these disorders. The efficacy and safety of onabotulinumtoxinA has been established in numerous randomized, placebo-controlled trials for more than 20 years, and although many studies have demonstrated the efficacy of onabotulinumtoxinA using standardized clinical outcome measures, there is a paucity of data on QoL as reported by the patient, particularly within the Canadian population. 1 Patient-reported outcomes have become increasingly important since the 1980s because they offer a way for the patient’s voice to be heard when assessing the quality and success of treatments. Regulatory bodies, reimbursement agencies, and third-party payers are ever more recognizing the need for patient-reported QoL data because clinical measures do not necessarily translate into real-world, meaningful functional outcomes as perceived by the patient.Reference Jankovic, Esquenazi, Fehlings, Freitag, Lang and Naumann 2 - Reference Wilson and Cleary 5 Physicians strive to improve patient’s QoL, which could in turn translate into better utilization of health care resources.Reference Grima, Torrance, Francis, Rice, Rosner and Lafortune 6
A multitude of tools, commonly referred to as utilities (which can be generic or disease specific and generally cover physical, mental, and social aspects) are available for assessing QoL.Reference Guyatt, Feeny and Patrick 7 - Reference Whitehead and Ali 9 This concept of using utilities is now widely recognized for interpreting QoL data in the medical community by deriving a score based on results from a patient’s self-reported questionnaire. Utility scores range between 0 and 1, with 0 indicating poor health and 1 indicating perfect health.Reference Parker, Moran, Roberts, Calvert and McCahon 10
The MOBILTY (MDs on BOTOX Utility; NCT00535938) project was designed to prospectively collect information in a large patient population from current Canadian practice to better understand the benefit of onabotulinumtoxinA treatment on a patients’ QoL over time across a variety of Health Canada–approved indications. Patients who were on maintenance onabotulinumtoxinA treatment were also included to allow comparison with those naïve to onabotulinumtoxinA treatment before entering the study.
Methods
Study Design and Population
MOBILITY was a prospective, multicentre, observational phase IV study conducted in Canada. Patients were consecutively considered for enrollment into the study between October 2007 and July 2012 at each centre if onabotulinumtoxinA treatment was deemed medically necessary. Patients could have been new to onabotulinumtoxinA treatment (naïve) or receiving ongoing treatments with onabotulinumtoxinA (maintenance). Participation in the study was limited to men and women aged ≥14 years who could provide written informed consent. Patients who had any contraindications to use onabotulinumtoxinA or were participating in any onabotulinumtoxinA clinical trial were excluded.
Baseline data were collected at the initial visit. Patients completed the Short Form 12 (SF-12) Health Survey before their first onabotulinumtoxinA injection on study. Additional SF-12 surveys were completed 4 weeks after baseline visit and at each subsequent study visit before treatment.
In addition to receiving onabotulinumtoxinA, there was no study-mandated intervention restriction once the patient was enrolled. Any treatment for a patient’s condition was able to be started, stopped, or changed as deemed appropriate by the physician. Safety and tolerability were monitored throughout the study period. Patients were considered to have completed the study if they completed at least five subsequent treatment visits. All data were collected on standardized case report forms and entered into an electronic database.
MOBILITY was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice, and the study protocol was approved by an independent ethics committee at each participating site. All patients were required to provide written informed consent before enrollment into the study.
Study Measures
The primary outcome measure was health utility (SF-6D), which was derived by converting patients’ QoL scores obtained from the SF-12 Health Survey, through the SF-6D Preference Based Algorithm.Reference Maruish 11 , Reference Ware, Kosinski and Keller 12 Additional data collected and reported here include demographics, onabotulinumtoxinA treatment history, and adverse events (AEs).
Statistical Analyses
The efficacy cohort, which included all patients enrolled per protocol who had a valid SF-6D score at baseline and at least one subsequent visit, was used to present the baseline demographic, clinical characteristics, and QoL data. The patient disposition and safety data are reported based on the enrolled cohort, which includes all patients who enrolled in the study and received at least one onabotulinumtoxinA treatment during the study. AEs considered treatment-related in the safety cohort included those reported as possible, probable, highly probable, or related by the investigator.
Because this project was exploratory in nature, no formal sample size calculations were carried out. Further, as this was an observational study, most analyses were descriptive in nature. Descriptive statistics and exploratory analyses of baseline and posttreatment outcomes data was performed using SAS software, version 9.2 (SAS Institute Inc., Cary, NC, USA). Regression analysis was carried out to obtain Loess smoothing plots for the health utility data. There were no hypotheses testing or p value calculations; however, 95% confidence intervals were provided around estimates of differences.
Results
Demographics and Disposition
A total of 1222 patients were enrolled into the study across 6 therapeutic indications and 46 different centres (Supplementary Appendix 1). Of the 1222 patients enrolled, 1062 were included in the efficacy analyses. The majority of the patients (n=697, 66%) were on maintenance onabotulinumtoxinA treatment at baseline. The most commonly enrolled indication was adult focal spasticity (AFS, n=398, 37%) and the least common was CP (n=22, 2%) (Table 1). A total of 38% (462/1222) of patients completed five subsequent visits. More maintenance patients (n=362, 47%) completed the study compared with those who were naïve to treatment at baseline (n=100, 22%). A small fraction of patients (n=118, 10%) was considered incomplete because they did not formally discontinue or complete the five required visits during the study. Patients who completed fewer than the five required subsequent visits and discontinued the study accounted for 53% (642/1222) of patients. Discontinuations because of AEs (including serious AEs) accounted for only 0.8% (5/642) of the discontinued population. Of the 642 discontinued patients, only 27 (4%) discontinued as a result of lack of drug efficacy, eight (1%) because of noncompliance, and eight (1%) from death. The most commonly reported reasons for study discontinuation included those categorized as “other” (337/642, 53%), loss to follow-up (177/642, 28%), and patient withdrawing consent (80/642, 13%). Study discontinuations categorized as “other” were mainly from the investigators discontinuing participation in the study (n=108/337, 32%) and the study ending before completion of the five required visits (n=106/337, 31%). Additional reasons included limited staff resources to complete the follow-up visit (n=34/337, 10%), patient moved or changed physician (n=25/337, 7%), patient decided not to continue treatment (n=21/337, 6%), or treatment was no longer needed (n=16/337, 5%). The remaining 27 patients (8%) reported various reasons, which were each reported in less than 2% of the discontinued population. Reasons for discontinuation between naïve and maintenance patients were generally comparable; however, minor differences were observed with respect to discontinuations because of lack of drug efficacy (naïve 5.3% vs maintenance 3.2%), noncompliance (naïve 2.3% vs maintenance 0.3%), and death (naïve 0.3% vs maintenance 2.1%).
Table 1 Distribution of patients in the efficacy cohort by indication
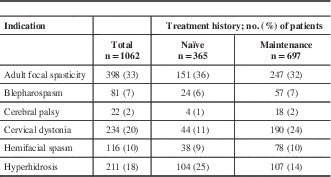
Baseline demographics of naïve and maintenance patients were generally comparable, with the majority being Caucasian (n=977, 92%) and female (n=675, 64%). Gender distribution was generally similar between naïve and maintenance patients for each indication (Table 2). Comparing across indications, hyperhidrosis patients had the highest frequency of full-time employment (n=131, 62%). Patient demographics within the population who completed the study was generally comparable with the efficacy cohort (Supplementary Appendix 2).
Table 2 Baseline patient characteristics stratified by indication and treatment history: efficacy cohort

CI, confidence interval; Diff, difference; maint, maintenance; NA, not available; SD=standard deviation.
* Unless otherwise indicated.
† Difference calculated for naïve minus maintenance groups, for means.
‡ Includes the following, which were each ≤5% of the population: Aboriginal, Arab/West Asian, Black, Chinese, Filipino, Japanese, Korean, Latin American, South Asian, South East Asian, and/or those categorized as “other,” except for the cerebral palsy cohort, in which South Asian accounted for 9% (n=2) of the maintenance population.
§ OnabotulinumtoxinA treatment characteristics before entering study; only applicable to maintenance patients.
Among the maintenance group, patients had received onabotulinumtoxinA treatment for their respective indications on average between 28 to 77 months before study entry. Blepharospasm, hemifacial spasm (HFS), and CD patients reported the longest average duration of onabotulinumtoxinA treatment before study entry, whereas hyperhidrosis, AFS, and CP patients reported shorter durations. On average, patients received 1.5 to 3.5 onabotulinumtoxinA treatments per year before beginning the study. Data from the patients who completed the study trended similarly.
Health Utility
With the exception of HFS, mean baseline SF-6D health utility scores were lower in naïve patients when compared with maintenance patients across indications in the efficacy cohort (Table 3). Maintenance patients exhibited modest improvements in health utility over the course of the study period, with the greatest mean change from baseline ranging between 0.000 and 0.036 for each indication. There was a trend towards a higher predicted SF-6D in naïve patients with the greatest mean change from baseline ranging between 0.019 and 0.066 for each indication. A greater improvement in scores from baseline was generally observed in naïve patients (Fig. 1). Although baseline scores were lower compared with maintenance patients, naïve patients within CD, blepharospasm, and hyperhidrosis demonstrated increases in health utility scores from baseline great enough to exceed scores of the corresponding maintenance patients by subsequent visit 5, whereas maintenance patient scores remained relatively stable or exhibited more conservative improvements. For most disease categories a greater initial response in health utility gain was observed in naïve patients as indicated by the steeper Loess health utility curve slopes during the first 4 to 6 months when compared with the maintenance group (Fig. 1). Overall, the Loess smoothing plots over time revealed the greatest benefit in health utility in the naïve CD population.

Figure 1 Longitudinal plot of SF-6D by treatment history across indications with Loess smoothing. Longitudinal plot for cerebral palsy cohort not presented because of low patient number.
Table 3 SF-6D Health Utility Scores Mean (±SD) change from baseline stratified by indication and treatment history: efficacy cohort

LTV, last treatment visit; SD, standard deviation; SV, subsequent visit.
* Data from n=1; single value reported.
At baseline, the majority of SF-6D domain scores (i.e. physical functioning, vitality, social functioning, role participation, mental health, and bodily pain) were higher in naïve patients compared with maintenance patients (Fig. 2), in which higher domain scores indicate poorer QoL. Among all indications, AFS, CP, and CD patients who were naïve to onabotulinumtoxinA treatment demonstrated the greatest impact of their disease on their QoL at baseline. In the maintenance cohort, the highest baseline SF-6D domain scores were observed in AFS patients, except in bodily pain, where cervical dystonia patients reported the greatest impact.

Figure 2 Mean baseline SF-6D domain scores by indication and treatment history.
In general, naïve patients exhibited improvements in their QoL throughout the study period, which was indicated by decreases in their SF-6D domain scores (Supplementary Appendix 3). In maintenance patients, the improvements in domain scores were modest and generally tended to remain near or fluctuate around baseline scores, across visits. Within the AFS and CD cohorts, naïve patients exhibited the greatest improvements in bodily pain and vitality domains. Bodily pain was the most improved domain in HFS patients, with notable improvements in role participation and social functioning domains at the earlier treatment visits. Blepharospasm patients who were naïve to onabotulinumtoxinA treatment had the most substantial decreases in social functioning and vitality domain scores, whereas naïve hyperhidrosis patients demonstrated the largest decreases in role participation and social functioning domain scores. Generally, the magnitude of the decreases in domain scores tended to wane by subsequent visit 4 or 5 in naïve patients. Overall, larger changes from baseline domain scores were observed in naïve patients compared with maintenance patients in the majority of domains by subsequent visit 5.
Among patients who completed the study (n=455/1062, 42.8%), baseline SF-6D health utility scores did not show any strong trends, whereas baseline domain scores were more often higher in maintenance patients versus naïve, with AFS patients having the highest baseline scores across the majority of the domains. In general, modest improvements in health utility were also observed within this population, with the greatest mean changes from baseline for each indication ranging between 0.003 and 0.097 for naïve and −0.008 to +0.039 for maintenance patients.
Safety
A total of 28 AEs in 18 of 1222 patients (2%) in the safety cohort were reported as treatment-related by the investigator (Table 4). Eight treatment-related serious AEs were reported in four patients, which included dysphagia (n=4), headache (n=1), local swelling (n=1), muscle tightness (n=1), and muscular weakness (n=1). The majority of the treatment-related serious AEs were reported in CD patients (n=7 events in three patients); only one case (dysphagia) was reported in one blepharospasm patient. The most commonly reported treatment-related AEs included muscular weakness (n=4) and dysphagia (n=5), each of which occurred in ≤1% of the overall safety population.
Table 4 Distribution of reported adverse events: safety cohort
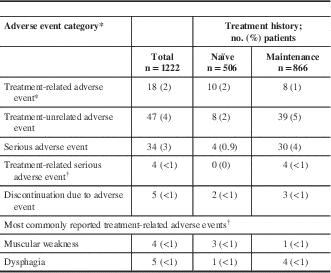
* Each patient is only counted once for each adverse event category.
† Includes cases categorized as related, highly probable, probable, and possible by the investigator.
Interpretation
To our knowledge, MOBILITY is the largest reported cohort receiving onabotulinumtoxinA in which QoL has been examined prospectively across a variety of indications. Our data demonstrate that, in many cases, QoL continues to improve after initiation of onabotulinumtoxinA in patient’s naïve to treatment. Another positive result with regards to those receiving maintenance onabotulinumtoxinA treatments is the preservation of QoL. This result contrasts a possible decline in QoL over time because of age, disease progression, or other contributing factors, in addition to the deterioration over time as would be expected with most chronic conditions.Reference Haacke, Althaus, Spottke, Siebert, Back and Dodel 13 - Reference Orpana, Ross, Feeny, McFarland, Bernier and Kaplan 16 Although the increases in QoL were not as pronounced in the maintenance population, a stable response and overall benefit in QoL was observed with long-term use, whereas naïve patients demonstrated more marked improvements in QoL, in particular in social functioning and bodily pain, following initiation of onabotulinumtoxinA. These results imply that onabotulinumtoxinA in clinical practice produces a substantial and early improvement in QoL that is sustained.
Among the numerous tools available to measure a patient’s QoL are the EuroQol-5D, health utilities index, and SF-6D. The SF-6D was selected for its applicability in a wide range of diseases and large patient populations while providing a number of useful descriptive health states.Reference Horsman, Furlong, Feeny and Torrance 17 , Reference Joore, Brunenberg and Nelemans 18 As expected, results from the SF-6D health utility evaluation demonstrated variation in baseline values across indications, illustrating the varying impact different diseases impart on QoL. Health utility values at baseline were generally lower in all naïve patients than in maintenance patients, suggesting an improvement in QoL resulting from long-term onabotulinumtoxinA use before study entry.
QoL in naïve patients showed clear improvement in the early stages of the study with the greatest improvements observed in cervical dystonia patients. The health utility values often continued to improve or were maintained throughout the study; however, the magnitude of the improvement tended to subside over time. The reduction in the number of patients over the course of the study may have resulted in this observed plateau. Yet, in many instances, naïve patient scores met or exceeded those of maintenance patients after several treatment cycles, which indicate that positive and sustained outcomes may require more than one injection. This is an important factor for both patients and physicians to consider when discussing treatment expectations during patient counseling. Not surprisingly, based on disease burden, naïve patients with HFS and hyperhidrosis exhibited the highest health utility scores over the course of the study, whereas AFS patients demonstrated the lowest.
In maintenance patients, health utility generally remained stable over the course of the study across indications. Given the length of treatment before study entry, long-term usage appears to result in maintenance of QoL, which is consistent with previously published reports on the long-term efficacy of onabotulinumtoxinA treatment.Reference Hsiung, Das, Ranawaya, Lafontaine and Suchowersky 19 - Reference Ramirez-Castaneda and Jankovic 21 The maintenance of QoL is an important finding because health utility naturally declines with age within the general population.Reference Hopman, Berger and Joseph 14 - Reference Orpana, Ross, Feeny, McFarland, Bernier and Kaplan 16 Furthermore, patients with disease etiologies such as stroke often experience more deterioration in their QoL over time.Reference Haacke, Althaus, Spottke, Siebert, Back and Dodel 13 , Reference McKevitt, Fudge and Redfern 22 , Reference Wolfe, Crichton and Heuschmann 23 Data from our study appear to indicate that treatment with onabotulinumtoxinA may be able to offset the natural decline and, at times, overcome it to result in continued improvements.
At baseline, QoL with respect to SF-6D domain scores was poorest in AFS, CP, and CD patients across all domains. Consistent again with etiology, hyperhidrosis patients were found to have the highest QoL at baseline. Maintenance patients exhibited modest improvements in domain scores across all domains at select time points, which is consistent with the SF-6D health utility findings that QoL was generally maintained over time. In contrast, patients who were naïve to onabotulinumtoxinA treatment exhibited pronounced improvements in social functioning, vitality, and bodily pain, which appear to be closely related to the disease characteristics. For instance, blepharospasm patients, who may be perceived as the most socially impaired because the condition affects their faces and may also affect vision and restrict mobility in some, reported having the greatest improvement in QoL with respect to social functioning and vitality domains. On the other hand, although the degree of visible neck posturing may impact social interactions, pain may be the more dominant consequence in CD patients, leading to more notable improvements in QoL with respect to bodily pain and vitality. As the results indicate, the assessment of QoL through examination of the separate domain scores provides more detailed information regarding the specific aspects of a patients’ QoL that may require more attention by the physician.
An analysis of data from patients who completed the five required treatment visits generally revealed similar results, with the greatest changes from baseline health utility scores observed in naïve patients versus maintenance. However, some differences were observed in the baseline health utility trends between the population of patients who completed the study and the efficacy population. Although these differences are acknowledged, it is difficult to interpret the significance of these trends because data were based on a substantially smaller population of patients. Data suggest that health utility was not a major contributor to patients’ study disposition because it would be expected that patients who completed the study may have achieved significantly better outcomes versus the total study population, yet outcomes were generally similar.
Overall, this study examined the long-term impact on QoL following treatment with onabotulinumtoxinA within a large patient population in Canada across a wide range of indications, providing a wealth of real-world patient data. The general trends reported here indicate that patients perceive an improvement or maintenance in their QoL following treatment with onabotulinumtoxinA. This appears to hold true even for maintenance patients who have had long-term exposure (up to 22 years before study entry) to onabotulinumtoxinA. Furthermore, even with long-term exposure to onabotulinumtoxinA, during the course of this study, the incidence of AEs remained low, which is consistent with previously reported safety data. 1 , Reference Hsiung, Das, Ranawaya, Lafontaine and Suchowersky 19 , Reference Ababneh, Cetinkaya and Kulwin 24 - Reference Rosales and Chua-Yap 27
Limitations and Strengths
A significant number of patients did not complete the five required subsequent injection visits, resulting in smaller analysis populations. The primary reason for the loss in patients was not from AEs, but was rather a consequence of the study closing before patient completion as well as site-related issues including the investigator discontinuing from the study, loss of the clinic nurse, and lack of patient follow-up. With this decrease in study population, data at the subsequent visits are not as robust for analysis. In addition, data may be further confounded by the patients’ use of concomitant medications or concurrent procedures, which was not prohibited in this study. Because treatments for many of these indications typically comprise several therapeutic components, it is recognized that data on QoL may also be influenced by these additional medications and/or procedures.
Although there are many outcome measures available, it is unclear which of these is best suited to assess the benefit of onabotulinumtoxinA injections across multiple indications. The SF-12 was selected because it is not disease-specific, which is an advantage for studying the heterogeneous population that received onabotulinumtoxinA treatment for a variety of indications; however, this lack of specificity may also be associated with limited sensitivity for the different indications, particularly when grouping these indications together.Reference Snyder, Aaronson and Choucair 28
With respect to patients specifically enrolled in the spasticity cohort, although the patient may suffer from focal spasticity, the underlying cause of the spasticity is quite heterogeneous (e.g. stroke, multiple sclerosis, spinal cord injury) and patients may have quite variable neurological function at baseline, which was not taken into account in the analysis presented here.
Overall, the design of the study allowed for incorporation of a variety of diseases with different severity, progression, and variable treatment history as well as dosing patterns. These factors, in addition to the imbalanced distribution of enrolled patients across indications, add confounding variability to the analysis and interpretation of data. Although these limitations exist, they also contribute to the strengths of the study. The goal was to report on outcomes from real-world clinical practice, which was successfully accomplished. By minimizing the controlling factors of the study, the data are expected to more accurately reflect real practice. In addition, the study was able to enroll patients across a number of indications allowing for cross-indication comparisons within a single study.
Conclusion
The MOBILITY study is the largest prospective study to date to provide patient-reported QoL data over a variety of indications following treatment with onabotulinumtoxinA. Although the QoL burden has been shown to vary by disease, data suggest that long-term treatment with onabotulinumtoxinA may help to improve or maintain health utility and QoL over time.
Acknowledgements and Funding
This study was sponsored by Allergan Inc. The authors thank Susan Simonyi of Allergan plc for her contributions to the design and execution of the study, Merlin Njoya of McKesson for statistical support, and Amy Kuang, PhD, of Allergan plc for editorial assistance during the development of this manuscript.
Disclosures
All named authors have contributed substantially to the interpretation of the data, first draft of the manuscript, and critical review of the manuscript for important intellectual content. All authors read and approved the final manuscript.
MJ has participated in speaking engagements and advisory boards with Allergan, Merz, Novartis, Abbvie, and Teva, and has received research grants from CIHR, AMOSO, MITACS, and NCE. TW has received research funds from Allergan, National Institutes of Health, and Boehringer Ingelheim; acted as a consultant for Allergan; and has received honoraria for accredited CME from Boehringer Ingelheim. MB and SD are full-time employees of Allergan plc. RM has been a member of a scientific advisory committee for Allergan and has been a speaker/consultant for Merz. FI has received research funds and honoraria for accredited CME from Allergan. RB has no conflicts of interest to disclose. Dr. Grace Trentin was formerly a full-time employee of Allergan plc during the conduct, analysis, and publication of this study and is currently a full-time employee of Novartis Pharmaceuticals Canada Inc.
Supplementary Material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/cjn.2016.262








