Manuscript
Brivaracetam (BRV) received Canadian approval for use as an adjunctive antiseizure medication (ASM) for focal epilepsy in adults in March 2016. Prior to its approval, three randomized controlled trials and a final meta-analysis established its effectiveness and assessed its adverse effects profile. Reference Ben-Menachem, Mameniskiene and Quarato1–Reference Ryvlin, Werhahn, Blaszczyk, Johnson and Lu4 These trials were limited to participants with frequent focal seizures (at least eight seizures in an 8-week baseline period) who were already undergoing treatment with one or two ASMs. Individuals with cerebral neoplasms, psychogenic non-epileptic seizures, or status epilepticus within the preceding 12 months were excluded.
While randomized controlled trials are considered the gold standard for evaluating intervention efficacy and are required for regulatory approval for a new medication, there are uncertainties regarding the generalizability of their results owing to their stringent exclusion criteria and generally short period of follow-up. Clinical trial results can also be influenced by the Hawthorne effect, where participants modify their behavior knowing that they are being carefully observed. Reference Delgado-Rodríguez and Llorca5 Descriptive and observational studies can address these issues, filling in knowledge gaps about the long-term efficacy and safety of medications.
Given that Canada was one of the first countries in which BRV was commercialized, it presents a valuable opportunity to conduct an observational study to assess BRV’s effectiveness and tolerability over an extended period. The primary objective of our study is to evaluate the effectiveness of BRV as a treatment for focal epilepsy in adults and systematically assess its tolerability.
We report our study according to the guidelines outlined by the STROBE statement. Reference von Elm, Altman, Egger, Pocock, Gøtzsche and Vandenbroucke6 This was a prospective observational cohort study. Study participants were adults (i.e., aged at least 18 years) with focal epilepsy followed by a neurologist in one of three participating sites and in whom BRV was to be added as an adjunctive ASM to treat their epilepsy. Individuals aged less than 18 years and individuals with generalized epilepsy were excluded from the study to respect Health Canada indications. Participants were recruited between October 2018 and March 2022 from three participating sites (Centre Hospitalier de l’Université de Montréal and Hôpital du Sacré-Coeur de Montréal in Quebec, QEII Health Sciences Centre in Nova Scotia).
We collected data during three successive clinical visits with the participants’ regular treating physician or nurse practitioner. Visits occurred at baseline and approximately 3 and 6 months. At baseline, we collected participant demographic and clinical characteristics, as well as patient-reported measures of irritability (measured using the Brief Irritability Test [BITe]), Reference Holtzman, O’Connor, Barata and Stewart7 anxiety (measured using the Generalized Anxiety Disorder–7[GAD-7] scale), Reference Spitzer, Kroenke, Williams and Löwe8 depression (measured using the Neurological Disorders Depression Inventory [NDDI-E] scale), Reference Gilliam, Barry, Hermann, Meador, Vahle and Kanner9 and quality of life (measured using the Quality of Life Inventory in Epilepsy-10 [QOLIE-10] scale). Reference Cramer, Perrine, Devinsky and Meador10 Baseline seizure frequency was determined as the number of seizures occurring in the month prior to study enrollment, as recalled by the participant or their next of kin. At every successive visit, we collected data on seizure frequency, changes in ASM, and adverse events.
We computed descriptive statistics for the entire sample, as well as for participants who participated at the 3-month and 6-month follow-ups. We report continuous variables as medians and interquartile ranges, and dichotomous variables as counts and proportions. Three-month follow-up data are provided in the Supplemental Material. We performed stratified analyses to assess the association between BRV dose, at baseline and at 6 months, and study outcomes at 6 months. We dichotomized baseline BRV doses and BRV doses at 6 months as follows: for baseline BRV doses, we stratified doses as low (< 100 mg/day) and high (≥ 100 mg/day); for 6-month follow-up BRV doses, we stratified doses as low (≤ 100 mg/day) and high (> 100 mg/day). We used a Mann–Whitney U test for continuous outcomes and a χ 2 test for dichotomous outcomes. We applied a statistical significance threshold of a = 0.05 for all tests. We did not correct for multiple comparisons as our goal was hypothesis generating rather than testing. We performed all analyses using R, version 4.0.2.
Informed consent was obtained from each participant prior to study enrollment. The study was approved by the Research Ethics Board of the Centre Hospitalier de l’Université de Montréal (# 18.032) as well as those of the two other participating sites.
A total of 51 participants were enrolled, among whom 38 completed the 6-month follow-up visit (Fig. 1). Participant baseline characteristics are detailed in Table 1. The median (interquartile range) age of participants at enrollment and at epilepsy onset were, respectively, 37 (30, 52) and 18 (8, 30) years. Half of participants were male and 82% had previously been on levetiracetam. The median (interquartile range) seizure count in the month prior to enrollment was 6 (3, 17).
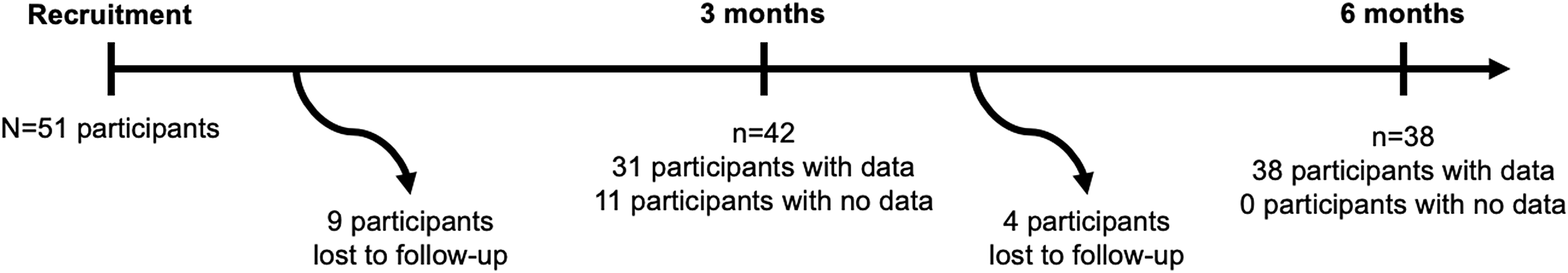
Figure 1: Flowchart diagram.
Table 1: Baseline characteristics
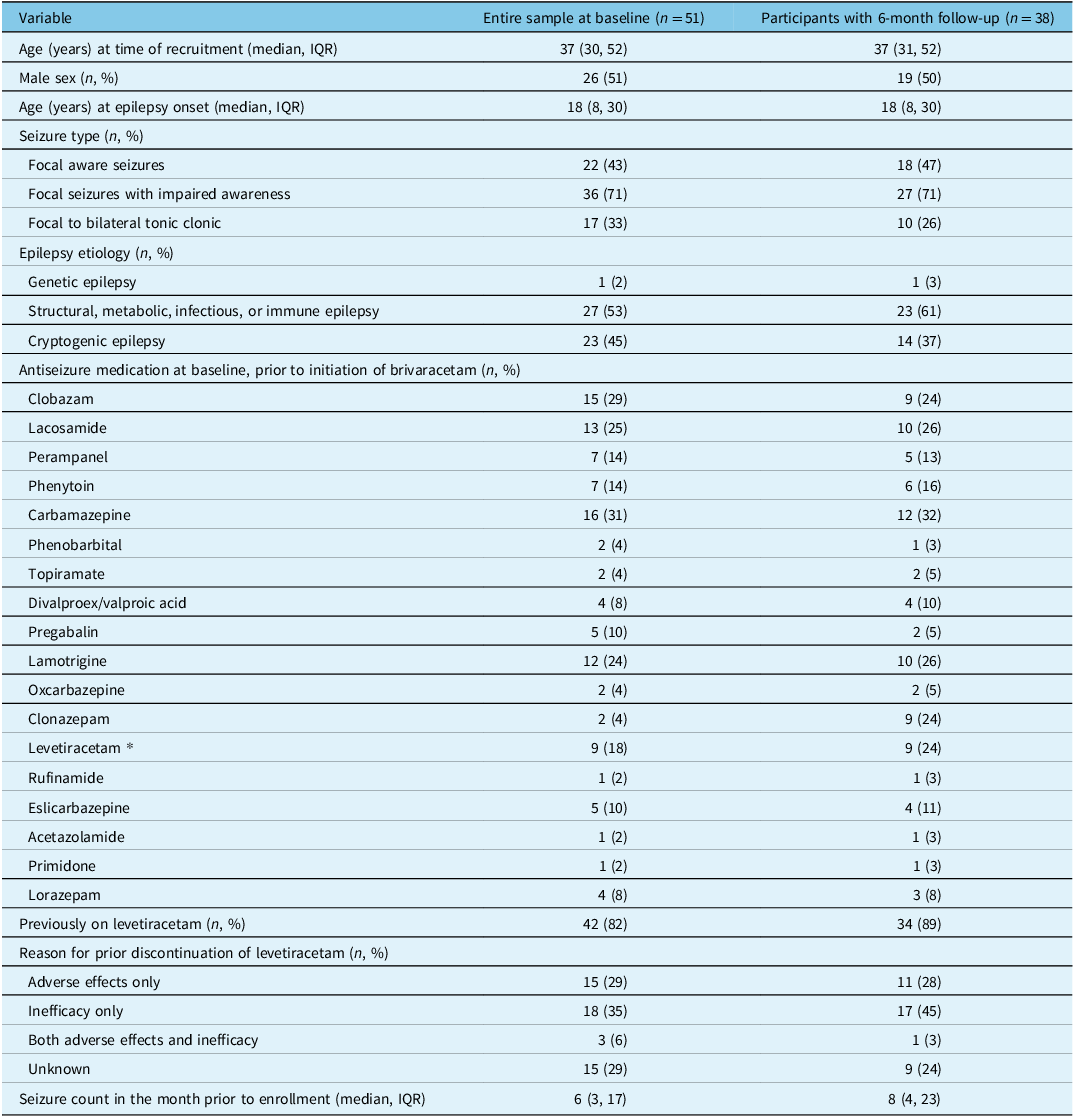
IQR = interquartile range.
* Leveteraticam was discontinued after initiation of brivaracetam.
At 6 months, 12 of 38 (32%) participants had discontinued BRV or increased the dose of another ASM. The remaining 68% of participants remained on BRV, with unchanged doses of their other ASMs. Among the 23 participants still receiving BRV at 6 months for whom data on seizure frequency at 6 months were available, 8 (35%) had attained seizure freedom (all but one of whom had been previously on levetiracetam), 11 (48%) had a ≥ 50% monthly seizure frequency reduction, and the median reduction in monthly seizure frequency was 40%. The proportion of participants with a ≥ 50% monthly seizure frequency reduction at 6 months was higher among participants taking a high BRV dose at 6 months than among participants taking a low BRV dose (73% vs. 25%, p = 0.02; Table 2). There were otherwise no significant differences at 6 months between participants who started with low versus high BRV doses (Table 3), or between participants who took a low versus a high BRV dose at 6 months (Table 2).
Table 2: Outcomes at 6 months stratified by BRV dose at 6 months
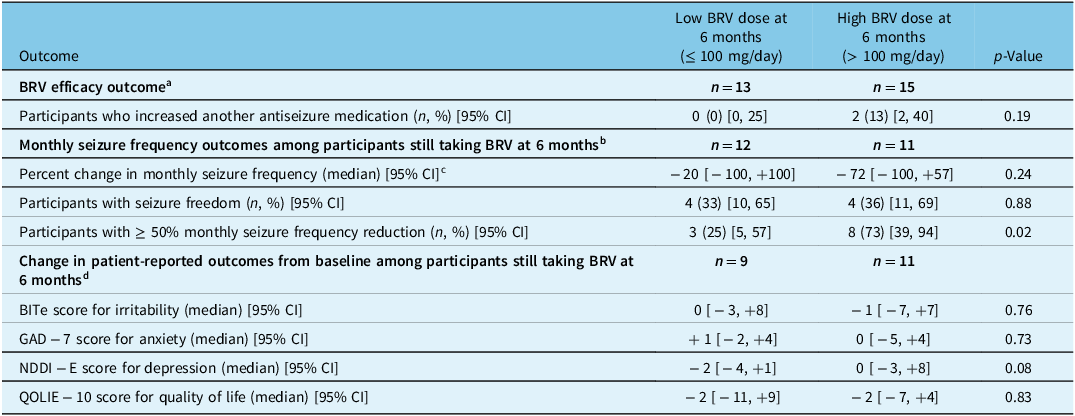
a Analysis of the BRV efficacy outcomes pertaining to discontinuing BRV were not performed, since participants who discontinued BRV do not have a 6-month BRV dose and therefore cannot be stratified.
b Among 28 participants still taking BRV at 6 months, 23 had data on seizure frequency at the 6-month follow-up.
c The number of participants contributing to this analysis was 11 for low dose and 10 for high dose, fewer than the number of participants with data on monthly seizure frequency since participants with 0 monthly seizures at baseline cannot contribute to percent change in monthly seizure frequency.
d Among 28 participants still taking BRV at 6 months, 20 had data on patient-reported outcomes at the 6-month follow-up.
Table 3: Outcomes at 6 months stratified by starting BRV dose
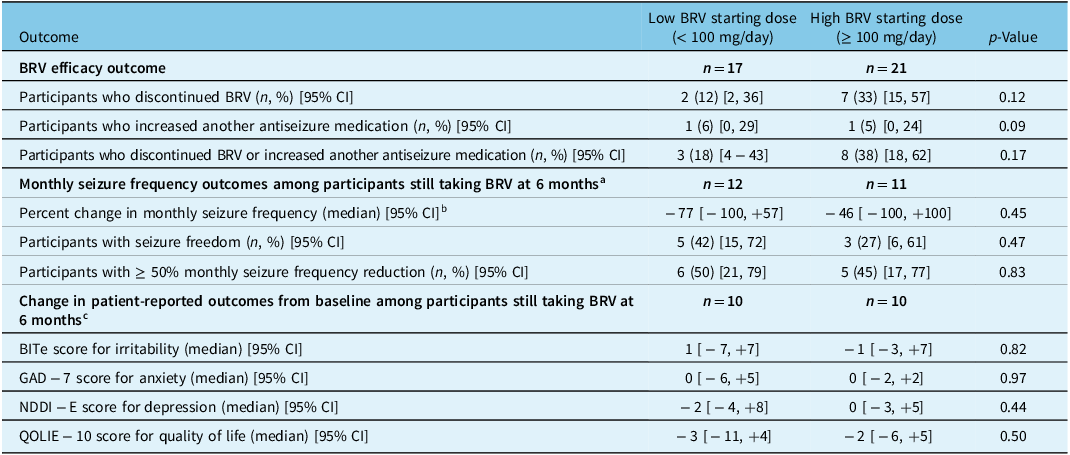
BRV = brivaracetam; 95% CI = 95% confidence interval.
a Among 28 participants still taking BRV at 6 months, 23 had data on seizure frequency at the 6-month follow-up.
b The number of participants contributing to this analysis was 11 for low dose and 10 for high dose, fewer than the number of participants with data on monthly seizure frequency since participants with 0 monthly seizures at baseline cannot contribute to percent change in monthly seizure frequency.
c Among 28 participants still taking BRV at 6 months, 20 had data on patient-reported outcomes at the 6-month follow-up.
We did not measure noticeable changes in BITe, NDDI-E, GAD-7, or QOLIE-10 scores between baseline and the 6-month follow-up among those still taking BRV, irrespective of starting dose or that at 6 months (Tables 2 and 3).
Our findings suggest that BRV is effective in reducing seizure frequency among adults with focal epilepsy. At 6 months, 68% continued BRV, while 32% had stopped it or the dose of another ASM was adjusted due to perceived inefficacy or intolerability. Thirty-five percent of participants still taking BRV attained seizure freedom, while 45% of participants saw their seizure frequency reduced by at least 50%. This efficacy was greater among individuals with high doses of BRV at 6 months, as compared to those with lower doses.
In the randomized clinical trials investigating BRV for focal epilepsy, Reference Biton, Berkovic, Abou-Khalil, Sperling, Johnson and Lu2–Reference Ryvlin, Werhahn, Blaszczyk, Johnson and Lu4 the pooled ≥ 50% responder rate were 34%, 40%, and 38% for BRV 50, 100, and 200 mg/day, respectively. Reference Ben-Menachem, Mameniskiene and Quarato1 Pooled results also found evidence of increased efficiency with higher maintenance BRV doses (≥ 100mg/day). Pooled seizure freedom rates were 3%, 5%, and 4% for BRV 50, 100, and 200 mg/day, respectively. In contrast to these results, our findings suggest that the benefit of BRV on seizure frequency reduction and particularly on seizure freedom might increase beyond the 15–18-week observation period reported in clinical trials.
For those remaining on BRV, patient-reported outcomes were unchanged at 6 months as compared to baseline, including measures of irritability and quality of life. We did not find evidence that the starting BRV dose was associated with symptoms of irritability, depression, or anxiety at 6 months.
Our study presents prospectively collected data from three medical centers in Canada on the efficacy and tolerability of BRV. Such “real-world” data allow for a portrait that is more generalizable that the original pivotal randomized controlled trials and their strict inclusion and exclusion criteria. We used validated measures for symptoms of irritability, depression, and anxiety, as well as quality of life. Our study has limitations, however. Our sample size was small due to difficulties in recruiting participants related to resource limitations. Between baseline and 6-month follow-up, 13 individuals were lost to follow-up. If these losses to follow-up were unrelated to whether a person was started on low- or high-dose BRV, or whether it was efficacious or well tolerated, we would expect these losses to bias our results toward the null. On the other hand, if these losses to follow-up were related to whether a person was started on low- or high-dose BRV, or whether it was efficacious or well tolerated, we would expect these losses to bias our results away from the null. Finally, who was treated with BRV, and the started dose, was decided on by the treating physician. This may lead to confounding bias, but such a study design also allows for a better appreciation of BRV utility in “real-world” clinical practice.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/cjn.2023.328.
Acknowledgments
The authors express gratitude to the study participants for their time.
Funding
This study was supported by a grant from UCB Canada for an investigator-initiated study. UCB Canada had no role in the design or conduct of the study, nor in the analysis of the data or writing of the manuscript.
Competing interests
MRK, SG, CJ, and DKN report unrestricted educational grants from UCB, Eisai, and Jazz Pharmaceuticals. MRK reports research grants for investigator-initiated studies from UCB and Eisai. DKN reports scientific presentation honoraria from UCB, educational grants from Sunovion, Liva Nova, Pendopharm, and Paladin, as well as fundraising contributions for clinical care and research from UCB, LivaNova, Sunovion, Paladin, and Jazz Pharmaceuticals. SG reports scientific presentation honoraria from UCB, Eisai, Sunovion, as well as for advisory board positions for Sunovion and Paladin Labs. CJ reports grant support from Epilepsy Canada. LBL reports speaking honoraria from UCB, Sunovion, and Eisai, advisory board honoraria for UCB and Sunovion, as well as a grant from CFI. RY, JNB, TDJN, MS, KI, JJ, and VC declare no competing interests.
Statement of authorship
Rayan Yanes and Joel Neves Briard are contributed equally.
MRK and DKN designed the study. RY, TDJN, MS, DKN, SG, KI, JJ, CJ, LBL, VC, and MRK carried out data collection. JNB and MRK performed data analysis. RY, JNB, and MRK drafted the manuscript. All authors reviewed the manuscript for intellectual content. MRK supervised the study.






