Introduction
Parkinson’s disease (PD) is characterized by prominent motor and various nonmotor symptoms associated with dopamine and other neurotransmitter dysfunctions. Motor symptoms such as asymmetric resting tremor, bradykinesia, rigidity, and responsiveness to dopaminergic agents are important clues to diagnose PD in clinical practice. Nigrostriatal dopaminergic system degeneration is known to be the main cause of clinical motor symptoms in PD. However, serotonergic dysfunctions are also associated with motor and nonmotor symptoms in PD, such as levodopa-induced dyskinesia (LID), depression, sleep disturbance, and fatigue.Reference Politis and Niccolini1 Its degeneration in PD has been found in previous animal, pathological, and neuroimaging studies,Reference Politis and Niccolini1 along with a 56% loss of serotonergic neurons in the median raphe nucleiReference Halliday, Blumbergs, Cotton, Blessing and Geffen2 and presence of Lewy neurites and Lewy bodies in the raphe nucleus at Braak stage 2.Reference Braak, Del Tredici, Rub, de Vos, Jansen Steur and Braak311C-3-amino-4-(2-dimethylaminomethylphenylsulfanyl)-benzonitrile (11C-DASB) positron emission tomography (PET) investigation of presynaptic serotonergic terminal function showed a reduced uptake in the striatum and extrastriatal areas.Reference Politis, Wu and Loane4 A reduction of serotonergic terminals was found to be correlated with disease duration.Reference Pagano, Niccolini, Fusar-Poli and Politis5 However, few studies have evaluated the differences in serotonergic activity according to age of onset and change in serotonergic activity after dopaminergic treatments.
The loudness dependence of auditory evoked potentials (LDAEP) is calculated using the amplitude of event-related potentials, such as N100 and P200 elicited by auditory stimuli (Figure 1). This has been used to evaluate central serotonergic activity in psychiatric disorders and PD.Reference Hegerl, Gallinat and Juckel6–Reference Beucke, Uhl and Plotkin8 It assumes that the serotonergic innervation is high in the primary auditory cortex.Reference Juckel9 Therefore, the LDAEP of a vertically oriented dipole, reflecting about 80% of activity of the primary auditory cortex, can be measured as the slope of the amplitude/loudness function in the N1/P2 potentials.Reference Hegerl, Gallinat and Juckel6,Reference Juckel9 Clinical and animal studies have indicated that the LDAEP is a reliable marker of central serotonergic activity, although some research involving humans has produced inconsistent findings.Reference Juckel, Molnár, Hegerl, Csépe and Karmos10,Reference Park, Lee, Kim and Bae11 LDAEP may be inversely correlated with central serotonergic activity.Reference Hegerl, Gallinat and Juckel6,Reference Juckel9,Reference Wutzler, Winter and Kitzrow12 High serotonergic activity may be demonstrated with a low LDAEP, while low serotonergic neurotransmission may be shown with a high LDAEP. In fact, a recent study found decreased serotonergic activity as well as dopaminergic activity in the early stages of PD using LDAEP.Reference Beucke, Uhl and Plotkin8
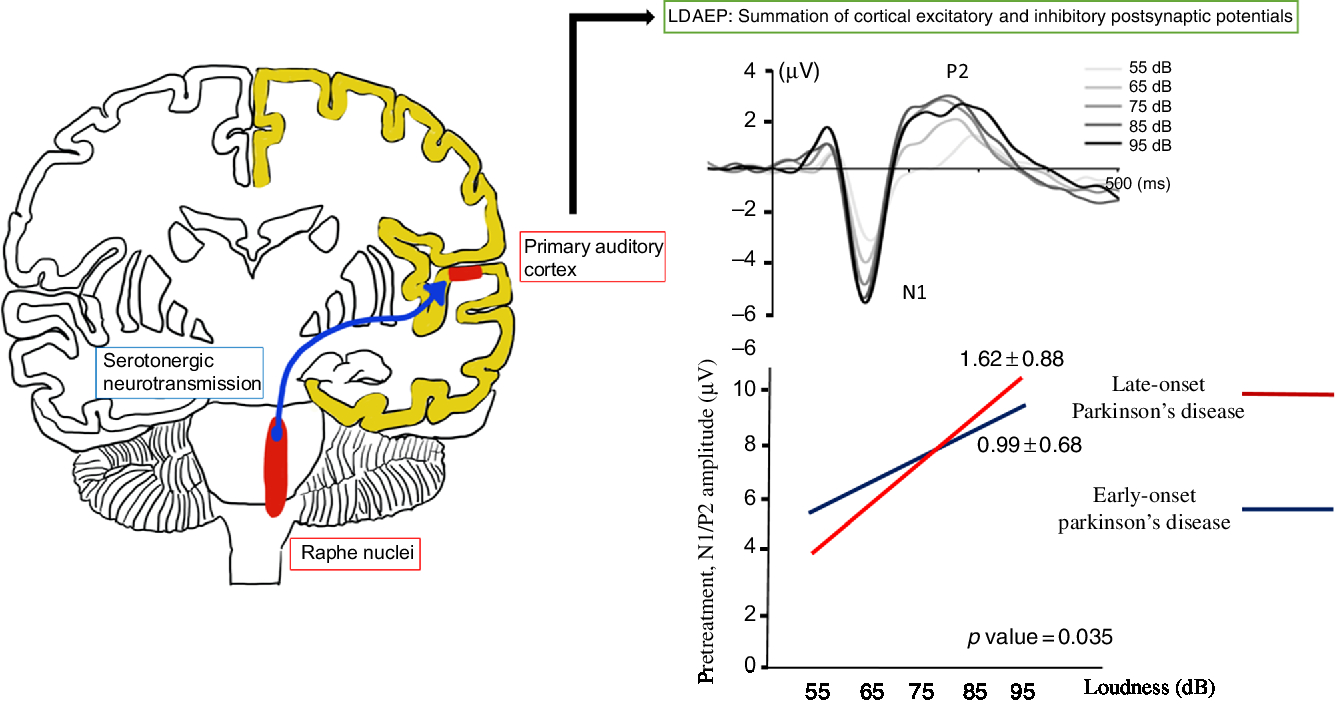
Figure 1: The loudness dependence of auditory evoked potentials (LDAEP) and the pretreatment N1/P2 LDAEP in late-onset Parkinson’s disease and early-onset Parkinson’s disease.
If LDAEP were a useful marker for central serotonergic activity in PD, preserved serotonergic activity would be shown in early-onset PD since early-onset PD has more frequent LID than late-onset PDReference Wickremaratchi, Ben-Shlomo and Morris13–Reference Mehanna, Moore, Hou, Sarwar and Lai15 and LID is known to be related to preserved serotonergic activity.Reference Pagano, Niccolini, Fusar-Poli and Politis5,Reference Björklund, Kirik, Carta and Carlsson16 This study aimed to determine central serotonergic functions in unmedicated PD patients using LDAEP to measure serotonergic activity by the disease itself and to compare serotonergic activity between early-onset PD and late-onset PD. Furthermore, since we assumed that serotonergic activity might be altered with dopaminergic medications in PD, we also investigated the differences in serotonergic activity depending on the use of dopaminergic medications.
Methods
Between September 2013 and January 2017, 30 patients with unmedicated PD, diagnosed based on the UK Brain Bank criteria,Reference Gibb and Lees17 were prospectively enrolled. Subjects with drug-induced parkinsonism, structural lesions causing parkinsonism, and serious medical illnesses were excluded from this study, along with subjects with major psychiatric disorders, including depression, diagnosed by a psychiatrist according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR). Only one patient had a family history of PD.
All participants were interviewed by a neurologist and underwent comprehensive neurological examination, laboratory analyses, brain magnetic resonance imaging, and LDAEP measurements. The levodopa equivalent dose was calculated.Reference Tomlinson, Stowe, Patel, Rick, Gray and Clarke18 Disease severity was assessed using the modified Hoehn and Yahr stage (modified H&Y stage), and motor status was evaluated using the Unified Parkinson’s Disease Rating Scale (UPDRS).Reference Fahn and Elton19 PD patients were initially divided into three groups: the young-onset group (≤49 years, n = 3), the middle-age group (50–69 years, n = 13), and the late-onset group (≥70 years, n = 14).Reference Mehanna, Moore, Hou, Sarwar and Lai15 However, since there were too few subjects in the young-onset group they were added to the middle-onset one, hence obtaining only two age groups: the early-onset group (≤69 years, n = 16) and the late-onset group (≥70 years, n = 14).
The N1 and P2 peaks were measured at the Cz electrode for auditory stimuli at five intensities (55, 65, 75, 85, 95 dB).Reference Lee, Park, Lee and Shim20 The LDAEP was measured twice, at baseline (pretreatment) and 12 weeks after administering a dopaminergic medication (post-treatment).Reference Lee, Park, Lee and Shim20 The LDAEP was determined by calculating the slope (the amplitude/loudness function) of the two N1/P2 peaks. Standardized low-resolution brain electromagnetic tomography (LORETA) was used to determine the current density in area BA41 (primary auditory cortex) using our previous method.Reference Lee, Park, Lee and Shim20
Ethics
The institutional review board approved this study (IRB subject no.: 2013-9-334), and informed consents were obtained from all participants.
Statistical Analysis
The Kolmogorov–Smirnov test was used to check whether the clinical variables were normally distributed. Student’s t-test, the Mann–Whitney U-test, and the χ 2 test were used to compare clinical variables between the early- and late-onset groups as well as between male and female patients. The pretreatment and post-treatment clinical variables in PD patients were compared by paired t-tests or Wilcoxon’s signed ranks test. Multiple binary logistic regression analysis for the relationship between age of onset and LDAEP was also conducted. All tests were two-tailed, and the cutoff for significant group differences was p < 0.05. The statistical analysis was performed using RexSoft.21
Results
Of the 30 patients with unmedicated PD, 66.7% were male. The mean age of the study population was 68.9 years and the mean age at onset was 67.2 years (Table 1). None of the patients had depression and none took antidepressants. Smoking was reported in one patient. Right-side-dominant parkinsonism was reported in 20 patients, while left-side-dominant parkinsonism was reported in 9 patients. Table 2 shows that there were no differences in demographic variables other than age and age at onset, or in the pretreatment LDAEP (N1 and P2), post-treatment LDAEP (P2 and N1/P2), pretreatment LORETA (N1, P2, and N1/P2), and post-treatment LORETA (N1, P2, and N1/P2) between the early-onset (n = 16) and late-onset (n = 14) groups. The absolute values of the pretreatment N1/P2 LDAEP (Figure 1) and post-treatment N1 LDAEP were significantly lower in the early-onset group than those in the late-onset group.
Table 1: Baseline demographics, LDAEP, and LORETA of PD patients
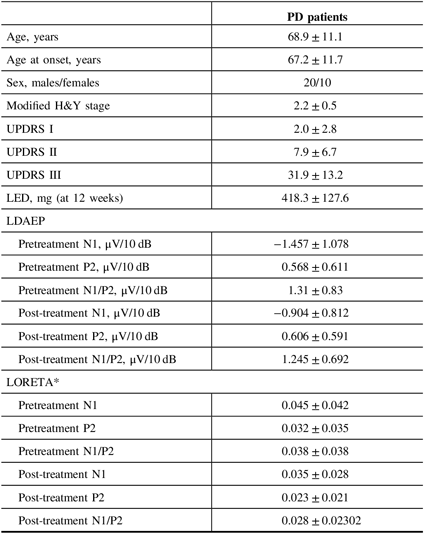
H&Y = Hoehn and Yahr stage; LDAEP = loudness dependence of auditory evoked potentials; LED = levodopa equivalent dose; LORETA = low-resolution brain electromagnetic tomography; UPDRS = Unified Parkinson’s Disease Rating Scale.
Mean ± standard deviation was presented.
* Wilcoxon’s signed ranks test.
Table 2: Comparison of demographic variables and LDAEP parameters between the early-onset and late-onset groups
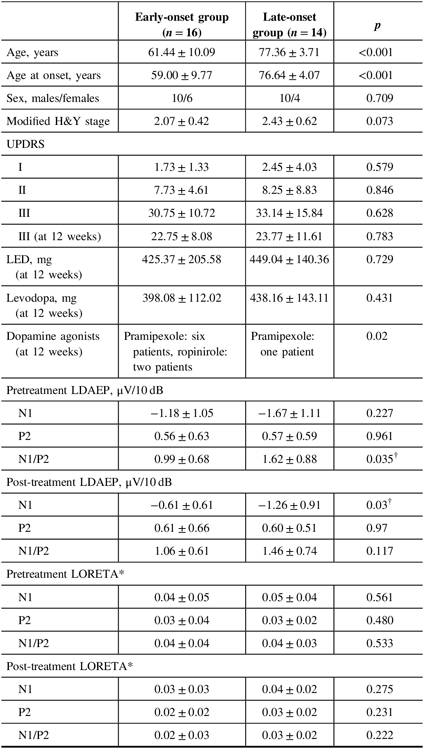
H&Y = Hoehn and Yahr stage; LDAEP = loudness dependence of auditory evoked potentials; LED = levodopa equivalent dose; LORETA = low-resolution brain electromagnetic tomography; UPDRS = Unified Parkinson’s Disease Rating Scale.
Data are mean ± standard deviation values.
* Mann–Whitney U-test.
† p < 0.05.
Demographic variables and the pretreatment LDAEP (N1, P2, and N1/P2), post-treatment LDAEP (N1, P2, and N1/P2), pretreatment LORETA (N1, P2, and N1/P2), and post-treatment LORETA (N1, P2, and N1/P2) did not differ between male and female patients. As shown in Figure 2, the post-treatment N1 LDAEP was significantly lower than the pretreatment N1 LDAEP after 12 weeks of taking a dopaminergic medication in all PD patients (p = 0.0018), in the early-onset group (p = 0.047), and in the late-onset group (p = 0.0072). There were no differences in the P2 LDAEP, N1/P2 LDAEP, N1 LORETA, P2 LORETA, and N1/P2 LORETA between pretreatment and post-treatment (Table 1). In addition, in multiple binary logistic regression analysis for the early- and late-onset groups, higher pretreatment N1/P2 LDAEP was significantly related with the late-onset group (coefficient = 1.204, p = 0.044) (Table 3).
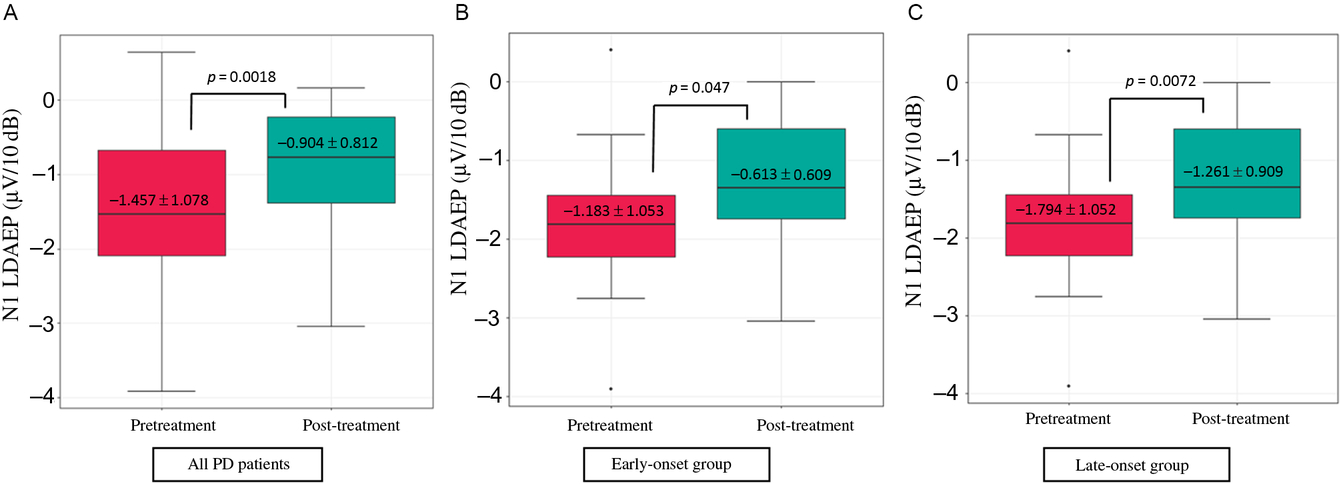
Figure 2: Change in the N1 LDAEP after 12 weeks of a dopaminergic medication. (A) Change in the N1 LDAEP in all 30 patients with PD. The absolute values of the N1 LDAEP in all patients decreased after 12 weeks of levodopa treatment (p = 0.0018). (B) Change in the N1 LDAEP in the early-onset group. The absolute values of the N1 LDAEP in the early-onset patients decreased significantly after 12 weeks of levodopa treatment (p = 0.047). (C) Change in the N1 LDAEP in the late-onset group. The N1 LDAEP in the late-onset patients decreased significantly after 12 weeks of levodopa treatment (p = 0.0072).
Table 3: Multiple binary logistic regression analysis for early- and late-onset groups
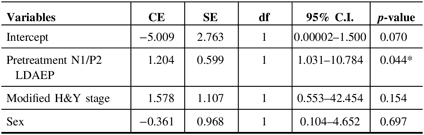
CE = coefficient; C.I. = confidence interval; df = degree of freedom; H&Y = Hoehn and Yahr stage; LDAEP = loudness dependence of auditory evoked potentials; SE = standard error.
* p < 0.05.
Discussion
This study found that the absolute values of pretreatment N1/P2 LDAEP and post-treatment N1 LDAEP were lower in the early-onset group than in the late-onset group. A higher pretreatment N1/P2 LDAEP was significantly correlated with the late-onset group. In addition, the post-treatment N1 LDAEP decreased significantly after 12 weeks of dopaminergic medications in all subjects, as well as in both the early- and late-onset groups. The smaller pretreatment N1/P2 LDAEP and post-treatment N1 LDAEP in the early-onset group suggests that central serotonergic activity was more preserved in this group than in the late-onset group. This effect was maintained after controlling for gender and disease severity (modified H&Y stage). The smaller pretreatment N1/P2 LDAEP was also related to early-onset PD based on multiple binary logistic regression analysis. These findings confirm our hypothesis that early-onset PD might have more preserved serotonergic activity than late-onset PD. Younger-onset PD patients are more likely to have LID,Reference Wickremaratchi, Ben-Shlomo and Morris13–Reference Mehanna, Moore, Hou, Sarwar and Lai15 a higher rate of dystonia,Reference Wickremaratchi, Ben-Shlomo and Morris13 and a slower progression,Reference Selikhova, Williams, Kempster, Holton, Revesz and Lees22 while older-onset PD patients are associated with more severe motor and nonmotor symptoms and a higher risk of dementia.Reference Wickremaratchi, Ben-Shlomo and Morris13,Reference Diederich, Moore, Leurgans, Chmura and Goetz23 In particular, LID in PD is known to be associated with serotonergic activity, which is due to excessive dopamine release in serotonergic terminals without autoregulatory feedback of dopamine release,Reference Björklund, Kirik, Carta and Carlsson16 and an aberrant sprouting of serotonin terminals could play a role in the development of LID in PD.Reference Rylander, Parent and O’Sullivan24 PD patients with LID showed preserved serotonergic terminals compared to PD patients without LID in a 11C-DASB PET study.Reference Pagano, Niccolini, Fusar-Poli and Politis5 Therefore, the higher serotonergic activity in the early-onset group in the present study is in accordance with results of previous studies, suggesting that LDAEP can be a useful noninvasive marker for central serotonergic activity in PD.
The N1 LDAEP was higher at pretreatment than after levodopa treatment, suggesting that serotonergic activity could be affected by dopaminergic medications as well as a disease per se. There is a growing body of evidence from animal, biochemical, postmortem, and human studies of loss of striatal and extrastriatal serotonin markers in PD patients suggesting that the serotonergic function is affected by PD pathology.Reference Politis and Niccolini1,Reference Maillet, Krack and Lhommee25 A previous study found the activation of the serotonergic system in rats with a lesioned striatum after levodopa had been infused.Reference Kääriäinen, García-Horsman, Piltonen, Huotari and Männistö26 Another study found that the LDAEP decreased after levodopa treatment in patients with PD,Reference Beucke, Uhl and Plotkin8 which is consistent with the finding of this study. The LDAEP was also significantly decreased by apomorphine, a dopamine agonist, in an animal study.Reference Juckel, Molnár, Hegerl, Csépe and Karmos10 These observations are associated with an increase of the serotonergic firing rate and release of serotonin in the raphe nuclei in the presence of dopamine agonists.Reference Kääriäinen, García-Horsman, Piltonen, Huotari and Männistö26 However, since the serotonergic activity was measured after a relatively short period in both the previous studyReference Beucke, Uhl and Plotkin8 and the present study in PD, caution is required when concluding that alterations of serotonergic activity might occur with dopaminergic medications. In contrast to the findings of this study, Politis et al.Reference Politis, Wu and Loane4 reported that 11C-DASB binding was not correlated with dopaminergic medications. Furthermore, a review article by Stansley and YamamotoReference Stansley and Yamamoto27 reported that an increased level of dopamine could damage serotonergic neurons via oxidative stress, and that chronic levodopa treatment could lead to deficits in the serotonergic system. Further studies are needed to determine the relationships between the serotonergic system and dopaminergic therapy since the effect of dopaminergic therapy on serotonergic activity may be influenced by various factors. These include the extent of neuronal loss in the serotonergic system or dopaminergic system, the duration or dosage of dopaminergic medications, and the combined use of serotonergic medications.
The main strengths of this study are the inclusion of unmedicated subjects, the investigation of serotonergic activity according to the age of onset, and the repeated measurements of LDAEP. The inclusion of 30 patients with de novo PD in this study made it possible to assess central serotonergic activity during the early stages of PD. This study divided the patients into two groups according to age at onset, which could reveal whether the age at onset affects different serotonergic functions. Lastly, the LDAEP was measured twice, before and after 12 weeks of taking a dopaminergic medication, thereby allowing any changes in serotonergic functions according to the use of dopaminergic medications to be detected.
Some limitations of this study are worth mentioning. Normal controls were not included in this study, which means that the effects of age on the central serotonergic neurotransmission could not be determined. This might influence the results of this study: a younger age onset could have more preserved serotonergic activities. The age criteria between the early- and late-onset groups are arbitrary. We used the criteria by Mehanna et al.,Reference Mehanna, Moore, Hou, Sarwar and Lai15 but the age criterion to classify groups according to the age of onset has been inconsistent. Although early-onset PD is more likely to be associated with LID, this study was not designed to evaluate LID since LDAEPs were examined in both unmedicated state and after 12 weeks of dopaminergic medications. Measuring the LDAEP does not assess the serotonin transporter or serotonin receptors but instead evaluates central serotonergic transmission in cortical areas. Thus, detailed information about the serotonergic system could not be obtained from the LDAEP measurements. However, these measurements are useful in clinical practice since they are both easy and inexpensive to perform. This study found that the serotonergic activity was higher in the early-onset PD patients and could be changed through dopaminergic therapy. Further studies are needed to confirm this finding.
Acknowledgements
We appreciate all the participants in this study. In addition, we are thankful to Dr. Irene Litvan (Department of Neurosciences, Parkinson and other movement centers, UC San Diego, La Jolla, CA, USA) for her contribution in preparing this manuscript.
Funding
This research was supported by a grant from the National Research Foundation of Korea (NRF) funded by the Ministry of Education (grant number: NRF-2018R1D1A1A02085847) and a grant from Research year of Inje University in 2016 (20150897). This research was also supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI18C0460).
Disclosures
HKP has received a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI18C0460).
J-JL declares no potential conflict of interest.
Y-MP has received a grant from the National Research Foundation of Korea (NRF) funded by the Ministry of Education (grant number: NRF-2018R1D1A1A02085847) and a grant from Research year of Inje University in 2016 (20150897).
Statement of Authorship
HKP wrote the manuscript; J-JL and Y-MP reviewed and edited the manuscript.







