Introduction
Placebo effects can be defined as beneficial effects generated by the context surrounding the administration of a treatment rather than due to the specific treatment itself. These effects depend on a complex interaction between internal factors such as the patient’s expectations and previous experiences associated with similar medical treatments and external factors such as environmental cues, and the patient–physician relationship. In clinical research trials, placebo responses are observed by participants in the placebo arm that are given an inert treatment, such as a sugar pill or sham device. This overall placebo response includes placebo effects and other nonspecific effects such as spontaneous improvement, regression to the mean, and Hawthorne effects (i.e., differences in performance/behavior by virtue of being observed in a trial).Reference Colloca and Barsky1 Despite not receiving active treatment, placebo responses can sometimes rival the effect sizes associated with medical treatments for some neuropsychiatric conditions.Reference Goetz, Wuu and McDermott2,Reference Polich, Iaccarino, Kaptchuk, Morales-Quezada and Zafonte3,Reference Jones, Razza and Weissman4 While placebo effects have long been viewed as a nuisance for clinical trials, research demonstrating activation of brain regions and networks by placebo effects has opened up a new line of scientific inquiry.Reference Benedetti, Carlino and Pollo5 This includes reconceptualizing placebo effects as a powerful neurobiological phenomenon that can be harnessed for clinical therapeutic applications. Furthermore, the evolving understanding of placebo effects has critical implications for trial design in clinical research, particularly with regard to neuromodulation.Reference Benedetti, Carlino and Pollo5,Reference Burke, Kaptchuk and Pascual-Leone6,Reference Burke, Romanella and Mencarelli7
HIGHLIGHT BOX: OPPORTUNITIES FOR ACTION
• Proper care in the trial design of placebo-controlled neuromodulation studies is essential to ensure valid scientific rationale, minimize risk, and properly attain informed consent.
• Although there are challenges with blinding neuromodulation, placebo-controlled studies must have adequate blinding with sham devices and procedures.
• To maximally combat challenges, adopt standardized methods and acquire blinding validity assessments.
• In the future, placebo effects may be better defined through using alternative placebo-informed trial designs.
Historically, the ethics of placebo groups in research trial design has been controversial due to concern of infringing upon patient autonomy by way of deception and by inadvertently posing harm due to not receiving the bona fide test treatment.Reference Emanuel and Miller8 Ethical considerations may differ depending on technological considerations, invasiveness, and other factors that may impact the trial design placebo group. Neuromodulation is a prime example of a field where trials cannot be properly blinded with a simple sugar pill and instead often require the development of elaborate sham devices or procedures for the placebo group. Neuromodulation approaches, such as transcranial direct current stimulation (TDCS), transcranial magnetic stimulation (TMS), electroconvulsive therapy (ECT) and magnetic seizure therapy (MST), vagus nerve stimulation (VNS), focused ultrasound (FUS), and deep brain stimulation (DBS), are becoming increasingly researched and used in the care pathways of many complex, and often treatment refractory, neuropsychiatric illnesses.Reference Williams, Taylor, Snipes, Short, Kantor and George9
Previous discussion on the intersection of placebo effects and neuromodulation has primarily focused on blinding considerations for sham devices and has been limited in scope.Reference Davis, Gold, Pascual-Leone and Bracewell10 In this article, we begin by reviewing the potential ethical issues that may arise in placebo-controlled research and apply these considerations toward neuromodulation treatment trials. We then discuss the unique aspects of placebo effects in neuromodulation that are important to be aware of, with an extended discussion on the importance of blinding effectiveness, and considerations of alternative trial designs. Finally, while an extended discussion on the mechanisms of placebo effects is beyond the scope of this review, we have briefly highlighted common misconceptions associated with placebo effects (Table 1). A summary of strengths and limitations of study designs for neuromodulation trials with a focus on placebo effects is also included (Table 2). Ethical considerations neurotechnology more broadly (e.g., neural interfaces, assistive technologies) is beyond the scope of this review and will not be discussed.
Table 1: Summary of common misconceptions of placebo effects and countering evidence

IBS = irritable bowel syndrome; OLP = open-label placebo.
Table 2: Strengths and limitations of study designs for neuromodulation trials with a focus on placebo effects
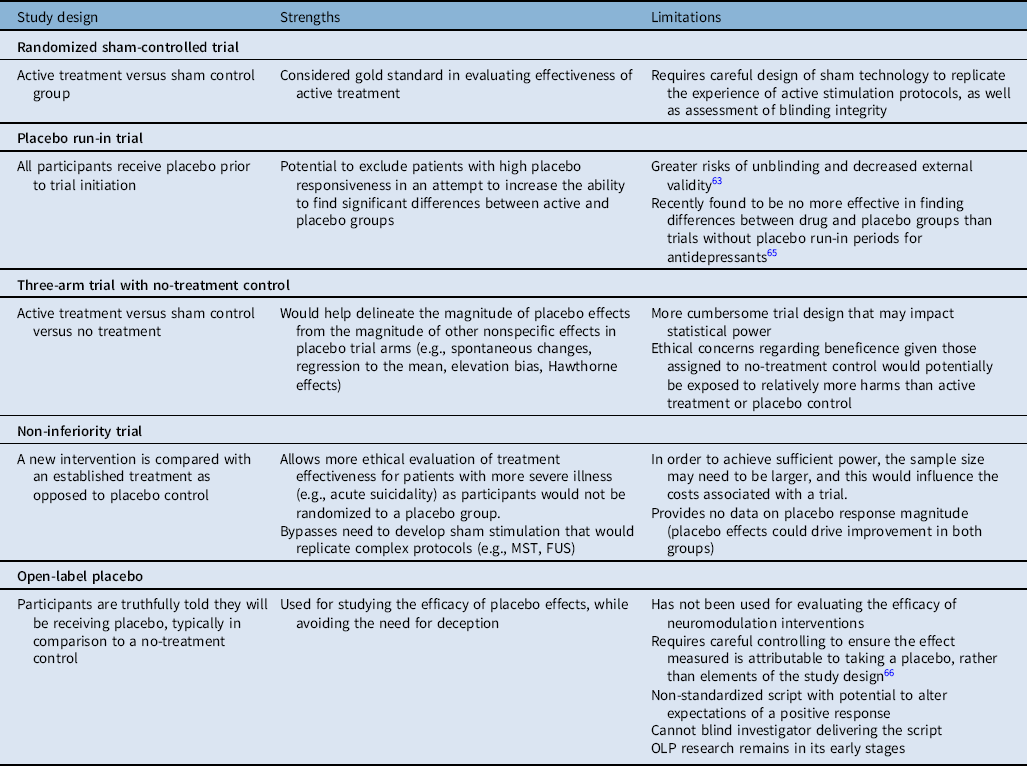
FUS = focused ultrasound; MST = magnetic seizure therapy; OLP = open-label placebo.
Case Study
Neuromodulation Research
Placebo-Control Group
Three overarching ethical principles govern clinical research: 1) autonomy and respect for persons, 2) beneficence, and 3) justice.Reference Miller, Crouch, Emanuel, Grady, Lie, Miller and Wendler24 Incorporation of a placebo-control group has been an essential component of advancing clinical research to determine effective treatments, and as such the methodology of neuromodulation trials with placebo/sham intervention must adhere to these ethical principles to minimize potential harm to patients.
Autonomy and respect for persons: Aspects of placebo-controlled research that may infringe upon autonomy and respect for persons include deception and an inadequate consent process.Reference Miller, Crouch, Emanuel, Grady, Lie, Miller and Wendler24 Patients randomized to placebo groups and discover they are not getting the test treatment could be disappointed or angered, and this may in turn undermine patient–doctor trust and cause undue influence on overall trial results.Reference Stoessl25,Reference Pugh, Kahane, Maslen and Savulescu26 Ways that deception can be minimized and deemed ethically justifiable are if patients are clearly advised in advance that they may be randomized into either active treatment or a placebo group, coupled with a protocol to debrief them as soon as their own individual participation is complete.Reference Pugh, Kahane, Maslen and Savulescu26 Another potential strategy to mitigate deception in research focused specifically on placebo effects is to use an open-label placebo (OLP) trial design (discussed in more detail below), whereby patients are truthfully told they will be receiving placebo.Reference Marks, Bartus and Siffert21 In all scenarios, each patient is owed a robust consent process outlining the risks of receiving placebo/sham intervention, such as forgoing active and potentially more effective treatment, periprocedural risks for more invasive neuromodulation (e.g., VNS, DBS) that still occur as part of the sham procedures, or both.Reference Stoessl25,Reference Pugh, Maslen and Savulescu27 Importantly, for certain vulnerable populations (e.g., children, intellectual disability, cognitive impairment), additional efforts to communicate placebo randomization and possible risks are required.
Beneficence: The goal of maximizing benefit and minimizing or eliminating harm is an essential principle guiding ethically justifiable research and medical practice. One ethical argument against placebo arms of trials is the perceived withholding of treatment to the patient, thus exposing them to harms of an untreated illness.Reference Pugh, Kahane, Maslen and Savulescu26,Reference Annoni28 For conditions with increasing severity and risk of mortality, such as refractory anorexia nervosa or acute suicidality, the implications of untreated illness would certainly be weighed differently.Reference Annoni28 However, inclusion criteria for neuromodulation interventions (particularly the more invasive interventions) typically require a level of treatment resistance, whereby several standard lines of intervention have been tried and failed.Reference Annoni28,Reference Burger, Capobianco and Lovern29 Another important consideration is that active treatment, particularly with surgical intervention, carries its own risks and adverse effects that need to be clearly communicated.Reference Annoni28
Use of a sham stimulation control group to evaluate efficacy of neuromodulation against serious conditions (e.g., acute suicidality) could be deemed ethically justifiable if appropriate monitoring (e.g., inpatient unit) and alternative treatment offered for non-responders are clearly outlined and offered, such as with recent trials of ketamine for acute suicidality.Reference Fitzgerald, Hoy and Elliot30 However, in some circumstances it may be more appropriate to use an equivalency, or non-inferiority trial design, whereby a new intervention is compared with an established treatment as opposed to placebo control, such as comparing the emerging MST with eECT,Reference Krishna, Sammartino and Rezai31 or FUS with DBS.Reference Lefaucheur, André-Obadia and Antal32 Also, methodology for certain neuromodulation trials (e.g., TMS) may suggest a washout period for medications in order to evaluate the true effect of the intervention.Reference Freedman33 In these instances, special care must be taken with close follow-up in place and a contingency plan in the event of acute clinical deterioration either before or during the intervention in order to minimize harms.
Arguments against the use of placebo as control in randomized trials primarily suggest that a breach of clinical equipoise may be occurring, and that comparing new interventions for conditions that have an established or standard treatment with placebo results in substandard, and thus unethical care.Reference Sinyor, Levitt and Cheung34 However, clinical practice for individual patients often differs from the goals of research, which is to determine safety, therapeutic efficacy, and potential generalizability of proposed interventions.Reference Stoessl25,Reference Burger, Capobianco and Lovern29 Furthermore, given findings of robust placebo responses for disorders such as treatment-resistant depression (large, pooled effect size [g = 1.05])Reference Jones, Razza and Weissman4 and in many neuromodulation trials more broadly,Reference Burke, Kaptchuk and Pascual-Leone6 being randomized to receive a placebo intervention would not be equivalent to having an illness be entirely untreated in a research trial. Although there would be important ethical considerations, a no-treatment group would help to better understand the magnitude of placebo effects in neuromodulation studies, which is important for determining the true efficacy of the active intervention.Reference Burke, Kaptchuk and Pascual-Leone6 A three-arm trial with active, placebo, and no-treatment control may thus be better suited for less invasive interventions with relatively lower disease severity so as to minimize the harms of the no-treatment group. Following a clear consent process and thoughtful trial design, patients randomized to no-treatment control should be offered active intervention after the shortest duration of time possible. Active treatment should also be offered to those randomized to the placebo group if found to be more effective.
Another important consideration pertaining to beneficence in placebo-controlled trial design is the “lessebo effect,” whereby there is a reduction in the magnitude of treatment effect in the active intervention group that is associated with the presence of a placebo group in the trial.Reference Mestre, Shah, Marras, Tomlinson and Lang35,Reference Kaptchuk36 This phenomenon may be due to the negative expectations associated with potentially being randomized to a placebo group.Reference Pugh, Kahane, Maslen and Savulescu26 To our knowledge, the lessebo effect has not been thoroughly evaluated in neuromodulation trials. The potential risk that the lessebo effect carries would be a Type II error (i.e., a failure to detect a significant difference between active and placebo intervention groups when one actually exists).Reference Pugh, Kahane, Maslen and Savulescu26 Whether, or how, this phenomenon impacts neuromodulation trials is unclear at this time and is a topic that necessitates further research.
Justice: As an ethical principle in clinical research, justice serves to uphold trust at the patient–doctor–-researcher interface and demands that all people receive equal and fair treatment.Reference Miller, Crouch, Emanuel, Grady, Lie, Miller and Wendler24 Recruitment for research trials must be without undue influence, and participants need to know they can withdraw without their medical care being affected. Given the potential for harms outlined above, particularly with more invasive neuromodulation interventions, it is imperative that the research question addressed is clinically important and will potentially result in a significant difference to clinical practice.Reference Annoni28 Furthermore, results of any publicly funded research must be disseminated and shared with not only the participants or subjects in the trial but also all of society, even if the results are those not expected or hoped for.Reference Miller, Crouch, Emanuel, Grady, Lie, Miller and Wendler24 Ensuring that appropriate and diverse groups are recruited in trials is an important tenet of justice in research design, as certain populations may be underrepresented. For example, indigenous or rural/remote communities often experience enormous systemic barriers accessing standard medical care. Unfortunately, neuromodulation trials mostly take place at tertiary academic care centers, which would result in underrepresentation of these populations and represents an important inequity to work toward overcoming in future trial designs.
Summary: For a randomized controlled trial (RCT) using neuromodulation with a sham stimulation group to be ethically justifiable, the trial must: 1) have a valid scientific rationale with clinical relevance, 2) ensure that potential risks do not outweigh potential benefits, and 3) involve informed consent with sufficient disclosure of information so that misconception of the purpose of the trial is avoided and the potential risks of being randomized to a placebo intervention group are clear and transparent (see Figure 1).Reference Stoessl25,Reference Pugh, Kahane, Maslen and Savulescu26,Reference Annoni28,Reference Burger, Capobianco and Lovern29

Figure 1: Ethical principles for the use of placebo controls in neuromodulation research trials. This figure was created using BioRender.
Placebo Effects
Many contextual factors modulate the magnitude of placebo effects. These factors can relate to the intensiveness of treatment, elaborate or innovative clinical settings, practitioner–patient relationships, societal perceptions (i.e., hype), and many other variables.Reference Olson, Lifshitz, Raz and Veissière37 Many of these factors are heightened in the setting of neuromodulation studies, which may inadvertently magnify placebo effects. For example, neuromodulation interventions are often inherently complex; they have lengthy procedures involving screening and calibration, sophisticated and expensive technology, and elaborate equipment requiring specialized technicians. The studies are often conducted in academic hospitals or laboratories filled with various credibility cues such as institutional logos, lab coats, and medical paraphernalia. The studies can involve lengthy discussions with physicians and researchers about the benefits and risks of the intervention, which provide the opportunity for positive and warm therapeutic relationships. Patients may even have developed positive expectations about the neuromodulation from seeing it featured in the media.
All of these factors combined can enhance placebo effects in the setting of therapeutic neuromodulation.Reference Burke, Kaptchuk and Pascual-Leone6,Reference Kaptchuk, Goldman, Stone and Stason38 To build on themes introduced above, elaborate and complex treatments tend to produce stronger placebo effects than simpler ones. Studies have shown that treatments involving acupuncture or medical devices tend to produce stronger effects than inert pills.Reference Kaptchuk, Stason and Davis39,Reference Meissner, Fässler and Rücker40,Reference Waber, Shiv, Carmon and Ariely41 Similarly, placebo procedures that appear more costly tend to be more effective.Reference Espay, Norris and Eliassen42,Reference Bernstein, Locher, Kube, Buergler, Stewart-Ferrer and Blease43 The various objects in the physical setting as well as the experimenter’s behavior can demonstrate cues of credibility and competence, which additionally can promote placebo effects.Reference Howe, Leibowitz and Crum44,Reference Howe, Goyer and Crum45 Lengthy discussions about the procedure can allow physicians to develop a connection with patients and demonstrate engagement and warmth, further boosting these effects.Reference Howe, Goyer and Crum45,Reference Faasse, Grey, Jordan, Garland and Petrie46 Also, expectations about the effectiveness of high-profile neuromodulation intervention can be modulated in participants who observe improvements of other patients.Reference Kaptchuk, Goldman, Stone and Stason38,Reference Faasse, Porsius, Faasse and Martin47,Reference Holtzheimer, Husain and Lisanby48
An additional complicating factor on this topic concerns the implications of potential shared neurobiological mechanisms between how placebo effects modulate the brain and how neuromodulation modulates the brain. A recent neuroimaging meta-analysis by Burke and colleagues identified a common set of brain regions demonstrating changes in activity when healthy individuals and patient populations experience placebo effects.Reference Burke, Romanella and Mencarelli7 This included activation of the left dorsolateral prefrontal cortex and the subgenual anterior cingulate cortex. They then showed that these activation clusters overlap with regions that are targeted by TMS and DBS to treat depression. There are many implications of the potential shared mechanism on conventional measurements of efficacy, and models of these impacts may help explain some of the variability in trial results that have been observed. For example, if placebo effects are particularly high (e.g., due to factors described above), the effect that TMS has on activating the dorsolateral prefrontal cortex brain target may effectively be stolen by placebo effects, which have already activated that brain circuit making it hard to show the incremental specific effect of TMS.
Blinding in placebo-controlled neuromodulation trials: Achieving satisfactory blinding in placebo-controlled neuromodulation trials is essential for proper assessment of placebo and treatment effects. Poor blinding may lead patients randomized to the placebo group to have decreased placebo effects by lowering participants’ expectations of a positive outcome.Reference Burke and Blumberger50 Conversely, poor blinding can also lead those randomized to the active group to have elevated placebo effects as they may have increased confidence and expectation that they are indeed receiving the active intervention.Reference Herwig, Cardenas-Morales, Connemann, Kammer and Schönfeldt-Lecuona51 This unequal distribution of placebo effects across trial arms can lead to major issues with interpreting clinical trial efficacy.Reference Herwig, Cardenas-Morales, Connemann, Kammer and Schönfeldt-Lecuona51 However, determining what constitutes adequate blinding in such trials is complex and varies by intervention studied. In the gold standard double-blind RCT, both participant and investigator are blinded to whether the participant is in the active or placebo arm. As most neurostimulation interventions are elaborate in nature (i.e., exposing patients to advanced machinery and complicated procedures), intricate sham controls are required to achieve even single blinding of the participant.
For noninvasive neurostimulation techniques including TMS, TDCS, and noninvasive vagus nerve stimulation (nVNS), blinded trials generally involve a sham control arm where the active treatment environment (e.g., appearance, sound, sensation) is mimicked, but little or no meaningful stimulation is received. For example, in TMS, specific sham coils have been developed that look indistinguishable from the active coil and make similar clicking sounds.Reference Davis, Gold, Pascual-Leone and Bracewell10,Reference Ambrus, Paulus and Antal52 In TDCS, pruritis under scalp electrodes may occur with stimulation onset or, less often, throughout treatment.Reference Davis, Gold, Pascual-Leone and Bracewell10,Reference Ambrus, Al-Moyed, Chaieb, Sarp, Antal and Paulus53 Sham controls may therefore involve brief activations of current to cause a similar feeling and pattern of itchiness.Reference Ambrus, Al-Moyed, Chaieb, Sarp, Antal and Paulus53,Reference Fonteneau, Mondino and Arns54 However, current sham TDCS protocols are heterogenous and may be confounded by separate neurobiological mechanisms enacted by seemingly inert brief activations from the electrodes, and further research is required to improve quality of sham procedures for TDCS.Reference Brunoni, Nitsche and Bolognini55 Active TDCS can also lead to local vasodilation and scalp redness that could be observed by raters, although sham stimulation over 30 seconds may also cause redness.Reference Schroeder, Möller and May56 To further improve blinding, many trials may rightfully exclude patients who have received previous treatment with a given neuromodulation as they will likely be able to note the difference of the sham procedures and experience.
Despite progress, there remain significant challenges to maintaining blinding integrity in neurostimulation trials. For instance, it can be challenging to reproduce the unpleasant scalp sensation and facial twitching that may occur with repetitive TMS.Reference Davis, Gold, Pascual-Leone and Bracewell10 There has also been debate whether tilting active coils off the scalp (a sham technique used in many early TMS studies) may be more susceptible to unblinding than sham controls.Reference Davis, Gold, Pascual-Leone and Bracewell10 To improve blinding, many trials may rightfully exclude patients who have received previous treatment with a given neuromodulation as they will likely be able to note the difference of the sham procedures. Along similar lines, conventional crossover design studies are generally discouraged as patients receiving active first may then be aware of the switch to sham. A final complicating factor is that in instances where the sham protocol involves low-dose stimulation, it could be argued whether the stimulation itself exerts an effect, a documented issue in previous nVNS trials.Reference Mestre, Lang and Okun57 In addition, operator blinding is generally very challenging, as the administrators must be familiar with the treatment protocols they are giving. Though novel protocols are trying to mitigate the impacts of this, it is important to have device administrators who are not involved in outcome evaluation. The lack of standardization and methodological heterogeneity sham protocols can impede assessment and meta-analyses of placebo responses across studies and between different treatment modalities.Reference Jones, Razza and Weissman4
Invasive neuromodulation techniques, such as DBS and VNS, also employ sham stimulation controls. While historically best medical treatment was commonly used as a control in DBS trials,Reference Bang, Flaherty, Kolahi and Park58 it would not control for the greater placebo responses expected in the DBS arm due to the elaborate nature of the intervention. In sham stimulation trials, the medical device being studied is inserted in both treatment and sham groups, but stimulation is only activated to therapeutic levels in the treatment group. This allows treatment crossover to occur, which largely circumvents ethical concerns associated with having an invasive sham surgery control. Blinding of a sham surgery would be additionally difficult in DBS, as patients are awake during the procedure. One challenge with the sham stimulation approach in DBS is the lesion effect, whereby clinical changes are derived from the lesion created by DBS lead placement itself. This can lead to a temporary physiologic effect in the sham group, as well as difficulty distinguishing stimulation benefit from the lesion effect. A strategy to combat this is by providing a washout period after device implantation where neither group is stimulated, thereby allowing time for the lesion effect to diminish. However, lesion effects are not entirely predictable; they have been shown to last months and likely vary depending on location and target symptoms.Reference Bang, Flaherty, Kolahi and Park58 Given the wide variety of illnesses under study for treatment with DBS and VNS, there are unique considerations for each condition that can impact blinding integrity. For instance, in trials of DBS for Parkinson’s disease, patients may require down-titration of anti-parkinsonian medications alongside DBS adjustments.Reference Bang, Flaherty, Kolahi and Park58 This could alert the patient to positive treatment response, unless a placebo pill is substituted.
The combination of elevated placebo effects and inherent challenges for blinding neuromodulation makes it critical to measure blinding validity in clinical trials. If blinding is inadequate, placebo effects can be erroneously attributed to the specific effects of the intervention and muddle the interpretation of trial results. Blinding validity is typically measured by determining whether participants and assessors can accurately deduce treatment allocation more often than by chance.Reference Schulz, Altman, Moher and Fergusson59 Assessing the success of blinding in placebo-controlled trials is a key step in evaluating internal validity that is frequently overlooked. For instance, a study by Fergusson et al. found that only 2% of RCTs reviewed in their analysis (of 191 trials) reported blinding validity in both participants and assessors/investigators.Reference Burke and Blumberger50 Unfortunately, in 2010, international clinical trial guidelines (CONSORT) removed requirements to measure blinding validity. Reasoning behind this decision included that such measures may rely on hunches on side effects or efficacy and even that patients may not answer truthfully.Reference Colagiuri and Benedetti60 This remains a controversial topic with many who oppose this position.Reference Shrout, Stadler and Lane61
Consistent evaluation and reporting of blinding success are critical to the proper evaluation of neuromodulation in clinical trial settings.
Placebo-informed trial designs for neuromodulation studies: In order to better characterize placebo effects in neuromodulation studies, alternative trial designs should be considered (see Table 2). Few prospective studies have been designed to research the potential differential placebo effects between types of treatment modalities, and none to our knowledge have done so in sham-controlled trials of neuromodulation.Reference Burke, Kaptchuk and Pascual-Leone6 Such a trial would include an active arm, sham stimulation arm, and additional placebo arm (e.g., an inert placebo pill). While this added placebo pill control would help characterize differential placebo responses, a no-treatment control group would be needed to delineate other nonspecific effects in placebo trial arms, including spontaneous changes, regression to the mean, elevation bias (whereby symptom severity is overreported at initial assessment),Reference McCambridge, Witton and Elbourne62 and the Hawthorne effect.Reference Laursen, Paludan-Müller and Hróbjartsson63 Though imperfect, the difference between responses in the placebo-control and no-treatment control would represent the magnitude of placebo effects distinct from the overall placebo response.
A potential strategy for reducing the large placebo effects observed in neuromodulation trials is by using a placebo run-in period. A placebo run-in includes a period at study onset where all participants receive a placebo, prior to randomization to active or placebo groups. The goal is to detect participants with high placebo responsiveness and exclude them from the trial. However, there are significant potential pitfalls of placebo run-ins, including risk of unblinding and decreased external validity.Reference Scott, Sharpe, Quinn and Colagiuri64 Furthermore, run-in trial design for antidepressants was recently found to be no more effective for finding differences between drug and placebo groups than trials without placebo run-in periods, and the authors advocated for cessation of run-in trial design in RCTs for antidepressants.Reference von Wernsdorff, Loef, Tuschen-Caffier and Schmidt65 Use of this debated protocol in neuromodulation trials should therefore be considered with caution.
As previously mentioned, an ethical consideration frequently encountered in the study of placebo effects is that of participant deception. This issue can be avoided in trials that use the OLP design. Emerging evidence from small randomized trials suggests placebo responses may persist even in the absence of deception, as seen in OLP trials for IBS,Reference Kaptchuk, Friedlander and Kelley15 back pain,Reference Carvalho, Caetano, Cunha, Rebouta, Kaptchuk and Kirsch16,Reference Zhou, Hall, Michaud, Blackmon, Partridge and Recklitis19 and cancer-related fatigue.Reference Kleine-Borgmann, Schmidt, Hellmann and Bingel18,Reference Kaptchuk and Miller20 Several recent reviews have addressed the current status of OLP studies in detail.Reference Kaptchuk and Miller20,Reference von Wernsdorff, Loef, Tuschen-Caffier and Schmidt65-Reference Colloca and Howick68 Briefly, the mechanism responsible for benefits seen in honestly administered placebos is poorly understood. Kaptchuk et al suggest that commonly proposed mechanisms of OLP response (e.g., expectation and conditioning) provide insufficient explanation, and that OLPs may act through disrupting central sensitization, abnormal signaling, and/or principles of the Bayesian brain.Reference Colloca and Howick68 There are several challenges to designing and evaluating OLPs. Careful controlling must occur to ensure the effect measured is attributable to taking a placebo, rather than elements of the study design (e.g., patient–provider interaction time).Reference Kaptchuk67 The script that explains the OLP concept to trial participants is not standardized, and different wording may alter expectations of a positive response. In addition, the investigator or clinician delivering the script cannot be blinded. Nonetheless, OLP research remains in its early stages and further investigation is warranted. To the best of our knowledge, OLP designs have not been used in neuromodulation studies. This could be a future direction for expanding the scope of OLPs studied.
Challenges and Opportunities
For placebo-controlled neuromodulation studies, care must be taken in trial design to ensure that the study has valid scientific rationale with clinical relevance, that steps are taken to prevent exposure to excessive risk, and that researchers provide informed consent with disclosure of information. Placebo responses in neuromodulation trials can be robust and efforts must be taken to try to ensure adequate blinding with sham devices and procedures. Given inherent challenges of blinding neuromodulation, standardized methods should be used, and blinding validity should be assessed to ensure proper interpretation of clinical trial results. Looking forward, alternative placebo-informed trial designs, such as the addition of a no-treatment control group, will better delineate the magnitude of placebo effects from other nonspecific effects in neuromodulation trials.
Conclusion
Considerations related to placebo effects have many important implications for the study and development of neuromodulation. Ethical justifiability of placebo-control groups in advancing neuromodulation research is contingent on adherence to three principles of ethical research: autonomy and respect for persons, beneficence, and justice. Vulnerability, trust, and meaningfulness of different procedures add a layer of complexity to an already complex ethical landscape, particularly when considering the need to incorporate marginalized and underrepresented populations in neuromodulation studies. Moreover, the risk-benefit ratio for different trial participants will change based on severity of illness and the invasiveness of the interventions.
Funding
Supported by the Liu Fu Yu Charity Foundation (MB).
Competing Interests
The authors have no competing interests to declare.





