Introduction
ATP-sensitive K+ (KATP) channels are located in various parts of the cell, including the surface of the plasmalemma (sKATP) and mitochondrial membrane (mitoKATP).Reference Bajgar, Seetharaman, Kowaltowski, Garlid and Paucek 1 KATP channels serve as metabolic sensors by providing a link between intracellular metabolic status and cellular excitability,Reference Nichols 2 and thereby participate in a variety of physiological functions. Activation of KATP channels with diazoxide can modulate neuronal excitability, regulate neurotransmitter release, and protect neurons from hypoxic/ischemic injury.Reference Sargent, Sleph, Dzwonczyk, Normandin, Antonaccio and Grover 3 - Reference Seino and Miki 5 MitoKATP channels are widely distributed in the brain; there are 6-7 times more mitoKATP channels per gram of mitochondria in cerebral cortex than in other organs (e.g., liver and heart muscle).Reference Bajgar, Seetharaman, Kowaltowski, Garlid and Paucek 1 Because the brain is rich in mitoKATP channels and diazoxide (DIZ) is considered to be a specific mitoKATP channel opener,Reference Garlid, Paucek and Yarov-Yarovoy 6 we deduced that mitoKATP channels play a significant role in regulating neurotransmitter release and neuronal excitability. Indeed, it is our opinion that mitoKATP channel function may be connected to GABAARs function or tonic currents.
Gamma-aminobutyric acid (GABA) is a primary inhibitory neurotransmitter in the central nervous system. Low concentrations of GABA in the extracellular space activate extra- or peri-synaptic GABAARs to generate persistent tonic inhibition. Tonic inhibition is involved in maintaining an inhibitory tone,Reference Ulrich 7 thus reducing the probability of action-potential firing.Reference Semyanov, Walker and Kullmann 8 Changes in neuronal excitability are associated with schizophrenia, epilepsy, and Parkinson’s disease.Reference Brickley and Mody 9 Extrasynaptic GABAARs are important elements of tonic currents. Gamma-5- and δ-subunit-containing GABAARs are primarily distributed in cortical layer 5.Reference Yamada, Furukawa, Ueno, Yamamoto and Fukuda 10 The neurons of layer 5 are for the most part large pyramidal cells with complex dendrites and contact with other neurons, where information undergoes sophisticated processing.Reference Drasbek and Jensen 11
In this study, we addressed the characteristic of tonic currents with development from cortical layer 5 pyramidal neurons in the developing rat brain and determined whether or not activation of KATP channels with DIZ regulates this developmental rule, whether or not the α5/δ-subunit-containing GABAARs participate in mediating the tonic currents, and if activation of KATP channels with DIZ exerts a modulating effect on the tonic currents with intervention of GABAAR. Our results are expected to provide deeper insights into the regulatory functions played by DIZ in cellular excitability and the potential role of mitoKATP channels as a novel therapeutic target for neuropsychiatric disorders related to GABAergic system dysfunction in the developing brain.
Materials and Methods
Ethics Statement
Our study was approved by the Ethical Committee of Animal Experiments at Zhejiang University School of Medicine, and all animal procedures complied with the guidelines of that committee. Our methods were carried out in accordance with the approved guidelines.
Preparation of Brain Slices
Male and female Sprague–Dawley rats were provided by the Experimental Animal Center of Zhejiang Province. The day of birth was considered as postnatal day 1 (P1). Rat pups were assigned to one of three or two postnatal age groups for experiments: P2–4, P7–8, and P30–34 groups; or P2–4 and P7–11 groups. For fresh brain slice preparation, rats were deeply anaesthetized with 10% chloral hydrate (380 mg/kg) by intraperitoneal injection and decapitated. The brain was rapidly removed and submerged in ice-cold and oxygenated (95% O2, 5% CO2) high-sucrose artificial cerebrospinal fluid (aCSF) (in mM): 150 sucrose, 50 NaCl, 25 NaHCO3, 10 dextrose, 2.5 KCl, 1 Na2HPO4.1H2O, 0.5 CaCl2, and 7 MgCl2; and then 300-μm thick coronal slices were prepared with a vibratome (Leica VT1000, Wetzlar, Germany). Slices were transferred to a nylon holder basket (AutoMate Scientific, Berkeley, California) immersed in carbogen-bubbled aCSF containing (in mM): 126 NaCl, 2.5 KCl, 1.25 NaH2PO4, 2 MgSO4 · 7H2O, 26 NaHCO3, 10 dextrose, and 2 CaCl2 at 35-37°C for ~60 min. This was followed by incubation at 22°C for ~60 min. All solutions were saturated with 95% O2/5% CO2.
Electrophysiology
The slices were transferred into a submersion-type recording chamber and superfused with oxygenated standard solution (32-33°C, 2-3 ml/min) and anchored with platinum wires on the bottom of the recording chamber. All recordings were obtained from layer 5 pyramidal cells visually identified using an infrared-sensitive CCD camera with a 63× water-immersion lens (Zeiss, Examiner A1, Oberkochen, Germany). Whole-cell patch-clamp recordings were carried out using a MultiClamp 700B amplifier controlled by pCLAMP 10 acquisition software (Molecular Devices, Sunnyvale, California). Currents were filtered at 1 kHz and sampled at 5 kHz using a Digidata 1440A (Molecular Devices). The recording pipettes were fabricated from borosilicate capillaries (OD: 1.5 mm; Warner Instruments, Hamden, Connecticut) using a Flaming/Brown micropipette puller (Model P-97; Sutter Instruments, Novato, California). We only performed whole-cell recordings if the initial seal resistance was ≥2 GS. For whole-cell recordings of tonic currents in the presence of exogenous GABA, patch electrodes (3-7 MΩ) were filled with KCl-based intracellular solution (in mM): 40 K-gluconate, 100 KCl, 2 NaCl, 10 HEPES, 4 EGTA, 4 MgATP, and 0.3 Na2GTP. For the voltage-clamp recordings, pyramidal neurons were held at −65 mV and bathed with 3 mM kynurenic acid (Tocris Bioscience, Avonmouth, Bristol, UK) to block glutamate receptors. Series resistance was measured after each recording, and data were discarded if the resistance changed by more than 20% or if the series resistance was >10 MΩ. The tonic GABAAR-mediated current was defined as the current blocked by SR95531 and measured as described.Reference Sebe, Looke-Stewart, Estrada and Baraban 12 To record tonic currents in the presence of exogenous GABA, slices were bath-perfused in GABA (5 μM; Sigma, St. Louis, Missouri) for 1-3 min followed by SR95531 (gabazine-selective specificity GABAARs inhibitors, 100 μM; Tocris) to block GABAARs. The GABAAR α5-subunit-selective inverse agonist L655708 (200 nM; Sigma) and δ-preferring agonist THIP (1 μM; Sigma)Reference Sebe, Looke-Stewart, Estrada and Baraban 12 were bath-applied following 1-2 min of 5 μM GABA, and followed by 100 μM SR95531. Slices were exposed to DIZ (100 μM; Sigma) and 5-hydroxydecanoate (5-HD; 100 μM; Sigma) for 3-5 min followed by SR95531. All perfused solutions were heated to 32-35°C by an in-line heater (Warner Instruments).
Data Analysis
To calculate statistical significance, we employed unpaired t tests and one- or two-way ANOVAs, depending on which test was appropriate for the dataset. The specific test used is noted in the text. Data are depicted as mean ± standard error of mean (SEM). Experimental values were considered significant if p<0.05.
Results
Tonic Current Decreases Dramatically with Postnatal Development
Figure 1 shows that the tonic currents markedly and rapidly decreased with development (Figure 1A,B). They decreased by 78.96% at P7–8 and by 85.67% at P30–34, respectively, which differed significantly at P2–4 (p<0.001); however, no significant difference was detected between P7–8 and P30–34 (p>0.05; Figure 1B). The resting membrane potential (RMP) increased with postnatal development. The level at P30–34 was significantly greater than that at P2–4 (p<0.05) (see Figure 1C). However, the input resistance was not significantly altered with postnatal development (see Figure 1D).
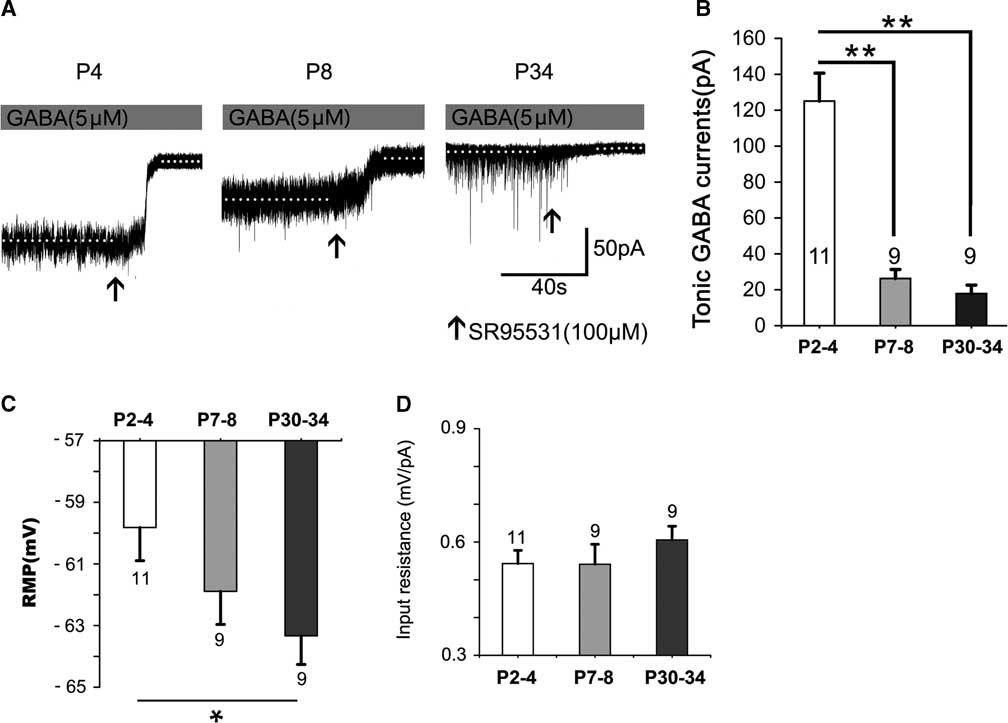
Figure 1 Tonic currents decrease dramatically with postnatal development. (A) Representative recording traces of the tonic currents from neocortical layer 5 pyramidal cells at P4, P8, and P34 with 5-μM GABA. (B) Bar graph shows the tonic currents recorded from neocortical layer 5 pyramidal cells at P2–4, P7–8, and P30–34. The tonic currents were robust in newborns and then decreased dramatically by the second postnatal week. The resting membrane potential (RMP) increased with postnatal development. The level at P30–34 is significantly greater than that at P2–4 (p<0.05) (see Figure 1C). However, the input resistance is not significantly altered with postnatal development (see Figure 1D). One-way ANOVA and least significant difference (LSD) post-hoc test. **p<0.001. Mean±SEM. Recordings were performed using KCl-based intracellular solution; Values within or above bars are the number of cells for the respective age group, as in Figures 2-5.
Opposing Effects of KATP Channel Opening on Tonic Currents at P2–4 versus P7–8 in the Presence of Exogenous GABA
Figure 2 shows that, compared to controls (see Figure 1), DIZ, a specific mitoKATP channel opener, inhibited the tonic currents at P2–4 and significantly enhanced the tonic currents at both P7–8 and P30–34 (one-way ANOVA, p<0.05) (Figure 2A,C). Lastly, the magnitude of calculated tonic current after adding DIZ to the aCSF was similar. Therefore, there was no significant effect among the three age groups administered DIZ (one-way ANOVA, least significant difference [LSD] post-hoc test, p>0.05). However, we did not detect an opposite effect on the tonic currents with 5-HD, a specific mitoKATP channel inhibitor, as treatment with 5-HD did not significantly alter tonic currents in any of the three age groups (Figure 2B,C). Furthermore, the baseline changes (ΔI hold) caused by DIZ affect the magnitude of the final tonic currents, and we found that DIZ resulted in a decrease of 59.08±7.36 pA at P2–4, and increases of 49.14±3.25 pA at P7–8 and 43.46±3.89 pA at P30–34, respectively (Figure 2A,D). However, GABA resulted in decreases of 117.16 ± 10.73 pA at P2–4, 94.84±18.65 pA at P7–8, and 78.57±12.18 pA at P30–34, respectively (Figure 2E).
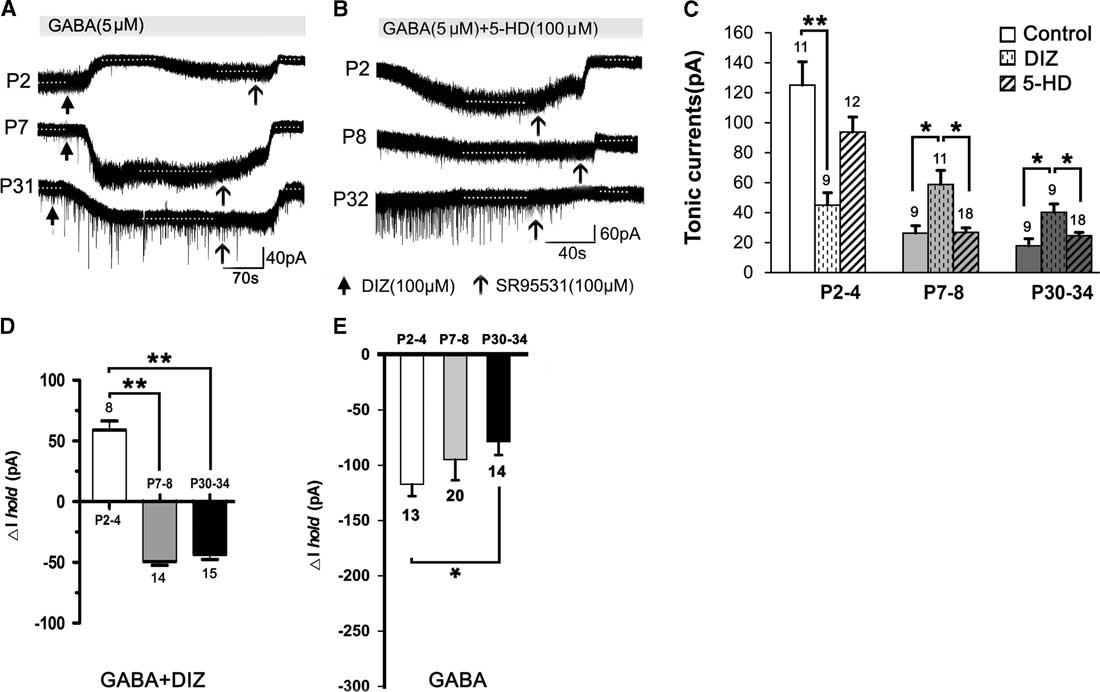
Figure 2 Opposing effects of KATP channel opening on tonic currents at P2–4 versus P7–8 and P30–34 in the presence of exogenous GABA. (A1,A2) Representative recording traces of the tonic currents at P2, P7, and P31 with DIZ, an opener of mitoKATP channels (A), and 5-HD, an inhibitor of mitoKATP channels (B). (C) Histograms show that DIZ inhibited the tonic currents at P2–4 and significantly enhanced tonic currents at both P7–8 and P30–34, compared to controls. However, treatment with 5-HD did not significantly change the tonic currents in the three age groups. Furthermore, DIZ caused an increase at P2–4 and decreases at P7–8 and P30–34 of the baseline changes (ΔIhold), respectively (D). Single application with GABA decreased ΔIhold for all three age groups (E). One-way ANOVA and LSD post-hoc test. *p<0.05, **p<0.001. Mean±SEM.
Opposing Effects of KATP Channel Opening on Tonic Currents at P2–4 versus P7–8
When recorded without exogenous GABA, DIZ also exerted similar effects. Figure 3 depicts how the magnitudes of the tonic currents at both P2–4 and P7–11 were smaller than in the presence of exogenous GABA (p<0.05; Figures 2B and 3A,B). The level at P2–4 was also significantly higher than that at P7–11 in controls (Figure 3B). Interestingly, we found that DIZ decreased the levels at P2–4 (p<0.05) and significantly increased them at P7–11 (p<0.05). In contrast, 5-HD did not significantly alter levels at either P2–4 or P7–11 (two-way ANOVAs, p>0.05; Figure 3B).
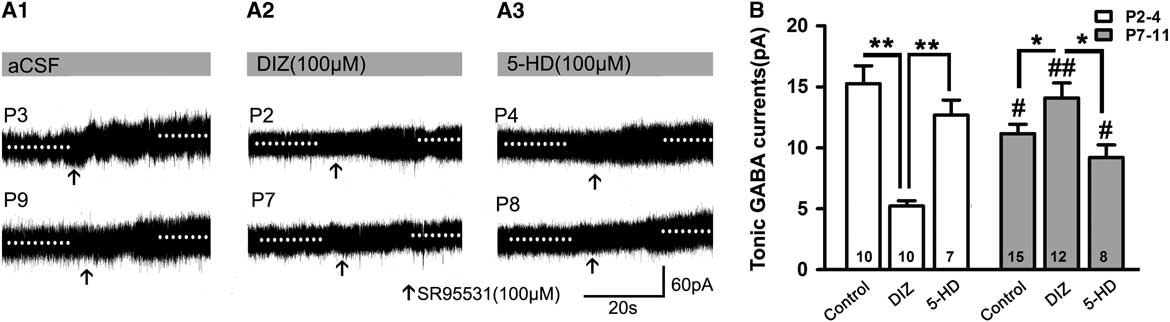
Figure 3 Opposing effects of KATP channel opening on tonic currents at P2–4 versus P7–8 when recorded without exogenous application of GABA. (A1–A3) Representative recording traces of tonic currents with aCSF (A1), DIZ (A2), and 5-HD (A3). (B) Bar graph indicates that the magnitude of tonic currents at both P2–4 and P7–11 are smaller than that with exogenous GABA. The level at P2–4 was significantly higher than that at P7–11 in controls, and DIZ decreased the level at P2–4 and significantly increased it at P7–11. In contrast, 5-HD did not significantly regulate its levels at both P2–4 and P7–11. Unpaired t test. * p<0.05, **p<0.001, #p<0.05, ##p<0.001 compared to P2–4 under the same conditions. Mean±SEM.
α5- and δ-Subunit-Containing GABAARs Participate in Mediating Tonic Currents in the Presence of Exogenous GABA
The α5,δ-containing GABAA receptors are rich in rat cortical layer 5; in L655708, an inverse agonist of α5-containing GABAARs; and THIP, a selective agonist of δ-containing GABAARs. We can record tonic current in the presence of THIP and/or L655708, which means that α5 and δ GABAAR take part in mediating tonic currents recorded in neonatal layer 5 neocortical pyramidal cells, and their contribution to tonic currents increases with development to some extent. This indicated that the expression of subunits α5 and δ and their influence on tonic currents were associated with rat age. THIP and L655708 increased ΔI hold, although the magnitude of tonic currents was small. We further analyzed ΔI hold caused by L655798 and THIP. The results were as follows: ΔI hold by 2.90±0.62 pA and 4.50±1.03 pA at P2–4, respectively; and by 3.98±0.76 pA and 3.82±0.58 pA at P7–11, respectively (Figure 4D).
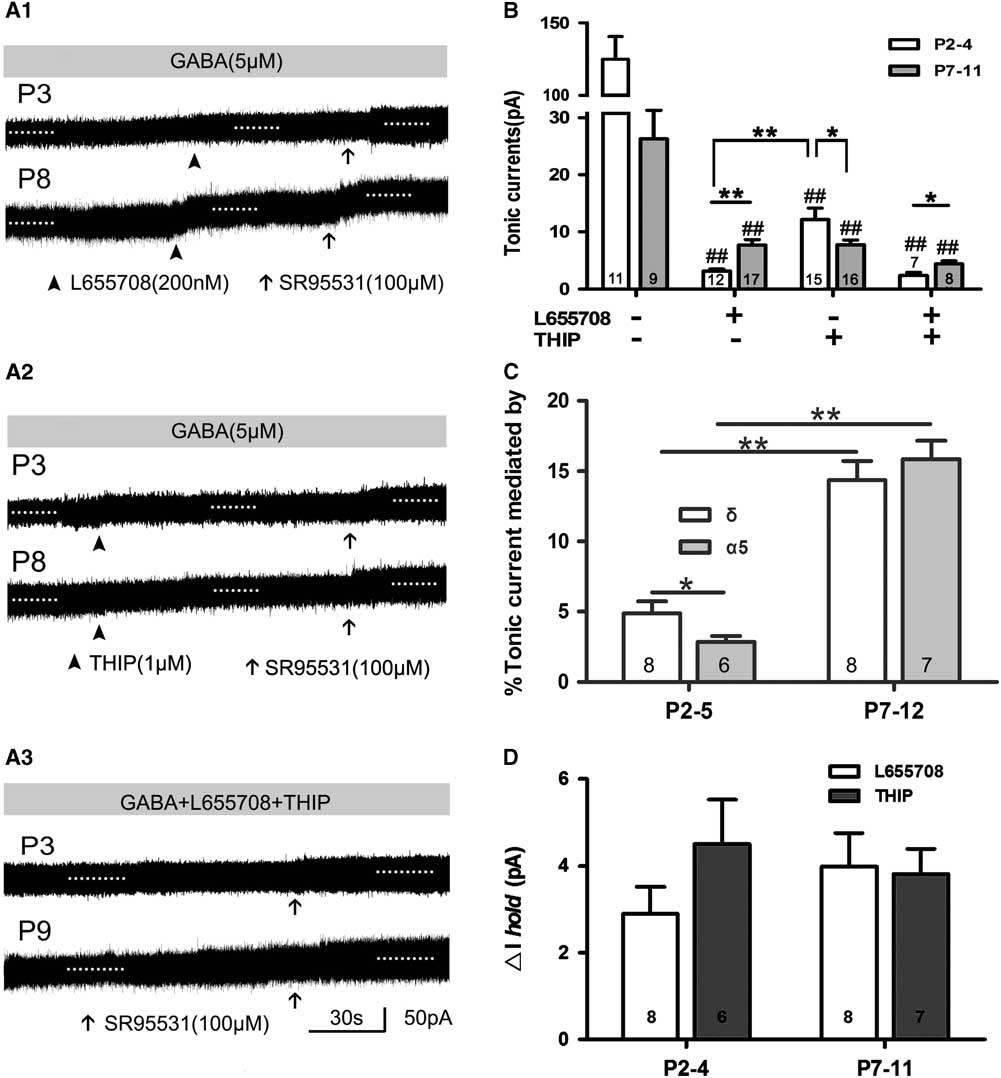
Figure 4 α5- and δ-subunit-containing GABAAR-mediated tonic GABA currents in the presence of exogenous GABA. (A1–A3) Representative recording traces of tonic currents with L655708, an inhibitor of α5-containing GABAARs (A1), THIP, an inhibitor of δ-containing GABAARs (A2), and L655708/THIP (A3). (B,C) Bar graph shows that α5 and δ GABAAR take part in mediating tonic currents recorded in neonatal layer 5 neocortical pyramidal cells, and that their contribution to tonic currents increased with development. *p<0.05, **p<0.001. Mean±SEM. One-way ANOVAs with post-hoc (LSD) analysis. (C) Bar graph indicates that α5 and δ inhibitor increased ΔIhold, although the magnitude of tonic current was small. Mean±SEM.
Opening KATP Channels Enhances Tonic GABA Currents with Application of L655708 or THIP
Opening of KATP channels with DIZ affected tonic currents in the presence of L655708 or THIP. We found that DIZ enhanced tonic currents in the presence of L655708 at P7–11 (Figure 5A1,B). DIZ also significantly increased tonic currents in the presence of THIP at P2–4 and P7–11, respectively, and this effect was most obvious at P2–4 (Figure 5A2,C). Our results indicated that opening of KATP channels with DIZ indeed mediates tonic currents.
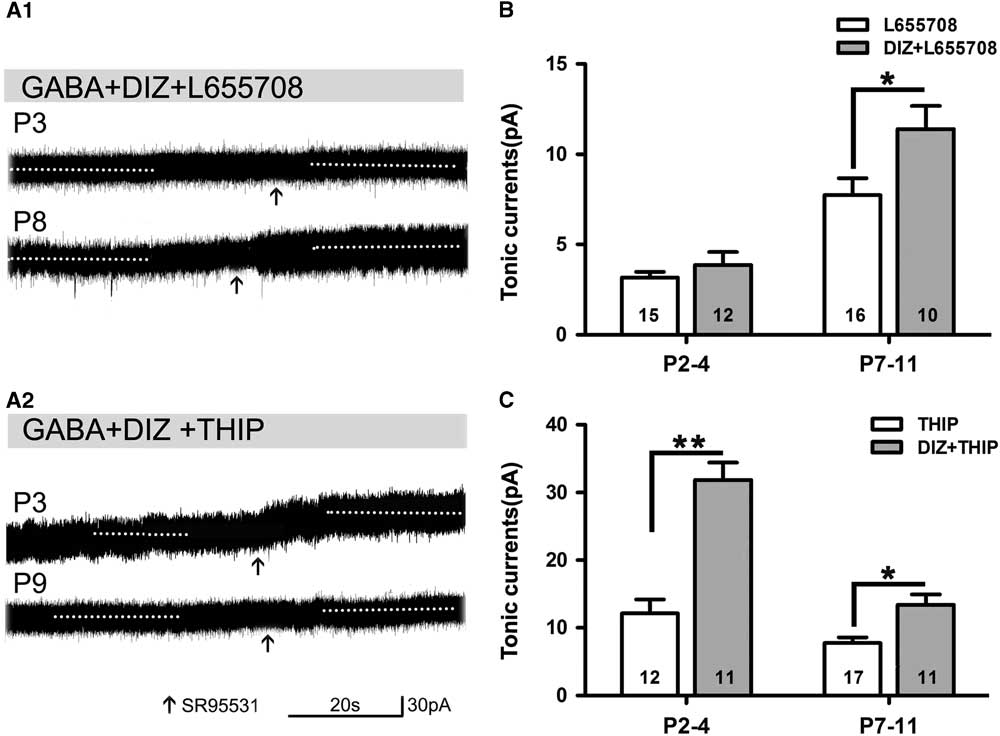
Figure 5 Opening of KATP channels enhances tonic currents in the presence of L655708 or THIP. (A1,A2) Representative recording traces of tonic currents under the conditions of mitoKATP channels activated by DIZ in the presence of L655708 (A1) and THIP (A2). (B) Bar graph shows that DIZ enhances tonic currents under the condition of α5 subunit- containing GABAAR blockade at both P2–4 and P7–11, but significant enhancement could only been detected at P7–11. (C) Bar graph indicates that DIZ significantly increases tonic currents under the conditions of δ-containing GABAAR blockade at P2–4 and P7–11, respectively, and this effect was obvious at P2–4. Unpaired t test. *p<0.05, **p<0.001. Mean±SEM.
Discussion
Elevated Tonic GABA Currents in the Presence of High Levels of Exogenous GABA
We found that the magnitude of tonic currents recorded after application of 5-μM exogenous GABA was higher than the tonic currents recorded without exogenous GABA at P2–4 and P7–11 (Figures 1A,B, and 3A,B). The magnitude recorded in the presence of 10-μM exogenous GABA (P3: 195.98 pA [n = 2]; P9: 37.25 pA [n = 3]) was also higher than the magnitude recorded with 5-μM exogenous GABA. Our finding that the tonic currents can be increased by elevated extracellular GABA concentrations implies that the GABAARs involved are not saturated by the ambient GABA in vivo because we showed that only a small portion of the para-synaptic GABAARs open at the same time, and this is an important feature of tonic currents in adult neurons.Reference Kasugai, Swinny, Roberts, Dalezios, Fukazawa, Sieghart, Shigemoto and Somogyi 13 Another possibility is that the increased GABA alters the relative contribution of specific GABAARs to the tonic currents, as other subunit-containing GABAAR populations may be recruited under conditions of increased ambient GABA.Reference Semyanov, Walker and Kullmann 8
Activation of KATP Channel-Regulated Tonic Currents in the Presence or Absence of Exogenous GABA
We found that DIZ exerts an opposite effect on the tonic currents recorded from cortical layer 5 pyramidal neurons during the first week versus the second week after birth; specifically, DIZ significantly decreased tonic currents at P2–4 but increased tonic currents at P7–10 (and also at P30–34). This regulation by DIZ might occur partially through GABAARs, and the ambient GABA concentration might be another key factor. Through the use of microdialysis techniques, investigators have demonstrated that KATP channels can regulate ambient GABA levels, and that DIZ dramatically decreases GABA levels in the ventromedial hypothalamus with a concomitant onset of hypoglycaemia.Reference Chan, Lawson, Zhu, Beverly and Sherwin 14 In the absence of glucose, KATP channel blockers enhance the release of GABA from rat hippocampal neurons.Reference Margaill, Miquet, Doble, Blanchard and Boireau 15 Although we detected DIZ-induced regulation of GABA release in the present study under relatively normal conditions and at certain levels of metabolic conditions in the aCSF, these metabolic factors may still be involved in the KATP channel’s effects on GABA concentrations. Within the hippocampus and neocortex, GABA is considered to be excitatory in early development due to a relatively depolarized Cl– reversal potential. It is possible that this opposite effect of DIZ on the tonic currents from cortical layer 5 pyramidal neurons may relate to this dynamic change in the action of GABA from excitatory to inhibitory with development.
Linkage of mitoKATP Channels to Alterations in Cellular Physiology
MitoKATP channels are distinct pharmacological targets. When cardiac mitoKATP channels and sKATP channels were studied under identical conditions, DIZ was shown to be a thousand times more potent in opening mitoKATP channels than sKATP channels. Further, 5-HD was shown to inhibit mitoKATP channels, but not sKATP channels,Reference Garlid, Paucek and Yarov-Yarovoy 6 , Reference Garlid, Paucek, Yarov-Yarovoy, Sun and Schindler 16 implying that the primary actions of DIZ are on mitoKATP channels. The fact that both DIZ and 5-HD failed to alter concentrations of nucleoside diphosphates and triphosphates when applied under normoxic conditionsReference Reinhardt, Manaenko and Guenther 17 suggests that there must be other factors involved.
The functions of mitoKATP channels are closely related to the mitochondrial K+ cycle. One distinct role played by the mitochondrial K+ cycle is to regulate the production of mitochondrial-reactive oxygen species for cell signalling.Reference Garlid, Dos Santos, Xie, Costa and Paucek 18 Our previous study showed that opening astrocytic mitoKATP channels with DIZ causes cytosolic K+ efflux through membrane gap junctions and pannexin channels,Reference Wang, Li and Feng 19 and this ultimately raises the extracellular K+ concentration, which can cause cell membrane hyperpolarization and reduce membrane excitability.Reference Kasugai, Swinny and Roberts 20
Effects of Activation of mitoKATP Channels on the Mean Baseline
The regulation of the tonic currents by mitoKATP channel activation could be related to effects on baseline holding currents, which were up-regulated at P2–4 and down-regulated at both P7–8 and P30–34 after DIZ administration in the presence of exogenous GABA. This change in baseline holding currents (ΔI hold) was negatively correlated with the magnitude of tonic currents under such conditions; however, this phenomenon was not observed without exogenous GABA, indicating that DIZ-induced regulation of tonic currents is related to ambient GABA concentrations. GABA caused a potent down-regulation of ΔI hold in the three age groups. Interestingly, activation of mitoKATP channels reverses this GABA-induced down-regulation of ΔI hold at P2–4, and diminishes the magnitude of ΔI hold at P7–10 and P30–34 (Figure 2D,E). Activation of mitoKATP channels with DIZ in the presence of exogenous GABA did affect baseline holding currents in the three age groups, and showed an opposite effect at P2–4 versus P7–10 and P30–34. This regulation by DIZ may be related to the dynamic changes in GABA from an excitatory to an inhibitory neurotransmitter with development.
Alpha-5,δ-Containing GABAARs Participate in Mediating Tonic Currents and Are Influenced by DIZ
Tonic currents are mainly composed of α5,δ-containing GABAARs in most brain regions under normal physiological conditions,Reference Brickley and Mody 9 and polymerase chain reaction results confirm that the α5 and δ GABAARs subunits are rich in cortical layer 5.Reference Yamada, Furukawa, Ueno, Yamamoto and Fukuda 10 We found that the α5- and δ-subunit-containing GABAARs take part in mediating tonic currents from neonatal pyramidal cells of somatosensory cortical layer 5, though the magnitude of the tonic current was small because research found that only a very small fraction of the available extrasynaptic GABAARs maintain simultaneous opening in adult neurons,Reference Kasugai, Swinny and Roberts 20 indicating that a large number of receptors are heavily desensitized.Reference Brickley and Mody 9 There is no direct link between DIZ/mitoKATP and GABAARs, but we found that DIZ can up-regulate tonic currents. We speculate that L655708 and THIP consumed α5- and δ-subunit-containing GABAARs, and other extrasynaptic GABAARs became sensitive to the GABA that existed in the bath solution, while mitoKATP activated by DIZ regulated GABA release under relatively normal conditions. Therefore, DIZ enhanced tonic currents in the presence of THIP and/or L655708.
Pathophysiological Implications
Previous studies have suggested that DIZ can prevent the initial hyperexcitability produced by anoxia,Reference Mattia, Nagao, Rogawski and Avoli 21 while according to our previous research we deduced that the anticonvulsant action of mitoKATP channels is achieved, at least in part, by enhancing astrocytic mitoKATP channel-regulated gap–junction couplingReference Jiang, Wang and Zhao 22 and electrical couplingReference Wang, Li and Feng 19 between astrocytes, in addition to neuronal mitoKATP channel-modulated excitability. The present study revealed a novel pharmacological profile of mitoKATP channel activators as regulators of tonic GABA transmission, and our results may provide a new strategy for antiepileptic treatments.
Conclusions
Tonic currents from neocortical layer 5 pyramidal neurons in rats follow certain rules with development. To some extent, the amplitude of the current depends on extracellular GABA concentrations. This neonatal tonic current was partially mediated by the GABAAR α5 and, likely, δ subunits. Activation of KATP channels, especially the mitoKATP channels, with DIZ has an opposite effect on the tonic currents with development.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (30570644 and 81300975), the Natural Science Foundation of Zhejiang Province (R2090266), the Zhejiang Province Key Technology Innovation Team (2010R50049), the Zhejiang Provincial Qianjiang Talent Plan (2011R10040), the Key Laboratory of Medical Neurobiology of the Ministry of Health at Zhejiang University, the Key Laboratory of Reproductive Genetics (Zhejiang University), the Ministry of Education, and the Key Laboratory for Diagnosis and Therapy of Neonatal Diseases of Zhejiang Province.
Disclosures
Dr. Li reports grants from the National Natural Science Foundation of China (81571263,81401071 and 81300975), the Natural Science Foundation of Zhejiang Province (R2090266), the Zhejiang Province Key Technology Innovation Team (2010R50049), the Zhejiang Provincial Qianjiang Talent Plan (2011R10040), the Key Laboratory of Medical Neurobiology of the Ministry of Health at Zhejiang University, the Key Laboratory of Reproductive Genetics (Zhejiang University), the Ministry of Education, and the Key Laboratory for Diagnosis and Therapy of Neonatal Diseases of Zhejiang Province, during the conduct of the study.
Dr. Wang reports grants from the National Natural Science Foundation of China (81571263,81401071 and 81300975), the Natural Science Foundation of Zhejiang Province (R2090266), the Zhejiang Province Key Technology Innovation Team (2010R50049), the Zhejiang Provincial Qianjiang Talent Plan (2011R10040), the Key Laboratory of Medical Neurobiology of the Ministry of Health at Zhejiang University, the Key Laboratory of Reproductive Genetics (Zhejiang University), the Ministry of Education, and the Key Laboratory for Diagnosis and Therapy of Neonatal Diseases of Zhejiang Province, during the conduct of the study.
Dr. Yu reports grants from the National Natural Science Foundation of China (81571263,81401071 and 81300975), the Natural Science Foundation of Zhejiang Province (R2090266), the Zhejiang Province Key Technology Innovation Team (2010R50049), the Zhejiang Provincial Qianjiang Talent Plan (2011R10040), the Key Laboratory of Medical Neurobiology of the Ministry of Health at Zhejiang University, the Key Laboratory of Reproductive Genetics (Zhejiang University), the Ministry of Education, and the Key Laboratory for Diagnosis and Therapy of Neonatal Diseases of Zhejiang Province, during the conduct of the study.
Dr. Jiang reports grants from the National Natural Science Foundation of China (81571263,81401071 and 81300975), the Natural Science Foundation of Zhejiang Province (R2090266), the Zhejiang Province Key Technology Innovation Team (2010R50049), the Zhejiang Provincial Qianjiang Talent Plan (2011R10040), the Key Laboratory of Medical Neurobiology of the Ministry of Health at Zhejiang University, the Key Laboratory of Reproductive Genetics (Zhejiang University), the Ministry of Education, and the Key Laboratory for Diagnosis and Therapy of Neonatal Diseases of Zhejiang Province, during the conduct of the study.







