Introduction
Migraine is a neurological disease characterized by frequent attacks with often incapacitating symptoms including headache pain, sensitivity to light and sound, and nausea. Migraine attacks can also be accompanied by prodromal symptoms followed by postdromal and interictal symptoms, which can be equally disabling for patients. Reference Dodick1 The International Classification of Headache Disorders, 3rd edition (ICHD-3) defines chronic migraine (CM) as ≥15 headache days/month for ≥3 months, with ≥8 headaches/month fulfilling the criteria for migraine with or without aura. 2 The overall prevalence of migraine diagnosed by a healthcare professional (HCP) in Canada is estimated at 8.3%, Reference Ramage-Morin and Gilmour3 with the prevalence of CM estimated at 392,000–600,000 based on the global prevalence of 1.4%–2.2%. Reference Natoli, Manack and Dean4,Reference Silberstein5 CM is associated with substantial disability and reduced health-related quality of life (HRQoL). Reference Burch, Buse and Lipton6–Reference Lanteri-Minet, Duru, Mudge and Cottrell8 In addition to the resulting burdens on families and society, Reference Buse, Fanning and Reed9,Reference Buse, Scher and Dodick10 CM is also associated with significant direct and indirect costs, including lost productive time at work, that lead to a large economic burden for individuals and healthcare systems. Reference Lanteri-Minet, Duru, Mudge and Cottrell8,Reference Hawkins, Wang and Rupnow11–Reference Stokes, Becker and Lipton13
Direct costs related to healthcare resource use and indirect costs such as lost productivity contribute to the substantial economic burden of CM to individuals and society. Reference Lanteri-Minet14 Individuals with CM report significantly more primary care visits, specialist visits, emergency room (ER) visits, and hospitalizations than those with episodic migraine (EM), defined as ≤14 headache days/month. Reference Katsarava, Buse, Manack and Lipton15 Evidence from the International Burden of Migraine Study showed that, in Canada, mean (SD) headache-related costs over 3 months were $471 ($1022) among individuals with CM compared to $172 ($920; p < 0.001) for those with EM. Reference Stokes, Becker and Lipton13 CM is also a leading cause of absenteeism Reference Baigi and Stewart16 and presenteeism in the workplace and exerts a toll on careers and finances. Reference Buse, Fanning and Reed9,Reference Stewart, Wood, Manack, Varon, Buse and Lipton17,Reference Serrano, Manack, Reed, Buse, Varon and Lipton18
Further exacerbating the individual and economic burden imposed by CM, individuals with CM may suffer from acute medication overuse (MO), a major cause of headache-related disability. The ICHD-3 defines MO headache as a headache occurring on ≥15 days/month in a patient with a pre-existing primary headache disorder that develops as a result of the intake of ergotamines, triptans, opioids, combination analgesics, or any combination of these medications on ≥10 days/month for >3 months or nonsteroidal anti-inflammatory drugs (NSAIDs) or acetaminophen on ≥15 days/month for 3 months. Data from the Canadian Headache Outpatient Registry and Database (CHORD) study found that 20.8% (125/606) of patients with CM referred to headache specialists were considered to have MO, defined as the use of opiates, ergotamines, or triptans on >2 days/week, non-opiate combination analgesics on >3 days/week, or paracetamol/NSAIDs on >5 days/week (note this definition is slightly different than ICHD-3 criteria). Reference Jelinski, Becker and Christie19 Diminished HRQoL and productivity loss are even greater in individuals with CM who also suffer from MO, Reference Lanteri-Minet, Duru, Mudge and Cottrell8 highlighting the importance of effective preventive treatment for CM to reduce the frequency of migraine attacks and thereby acute medication use.
OnabotulinumtoxinA (Botox®; Allergan, an AbbVie Company) is approved worldwide for preventive treatment of CM in adults. Both clinical trials and real-world studies have demonstrated the effectiveness of onabotulinumtoxinA to reduce headache frequency and severity and improve HRQoL Reference Dodick, Turkel and DeGryse20–Reference Frampton and Silberstein24 in individuals with or without MO. Reference Negro, Curto, Lionetto, Crialesi and Martelletti25,Reference Silberstein, Blumenfeld and Cady26 Additionally, onabotulinumtoxinA treatment improves work productivity Reference Stark, Stark and Limberg27,Reference Blumenfeld, Patel, Turner, Mullin, Manack Adams and Rothrock28 and reduces disability and real-world healthcare resource utilization (HRU) in adults with CM in the USA and Europe. Reference Rothrock, Bloudek, Houle, Andress-Rothrock and Varon29–Reference Rothrock, Stark, Sommer and Blumenfeld33
OnabotulinumtoxinA was first approved for preventive treatment of CM in Canada in 2011 but until recently, there was limited real-world evidence on the effects of onabotulinumtoxinA treatment for CM within Canada. Results from the Canadian, multicenter, prospective, observational, standard of care PREDICT study demonstrated that onabotulinumtoxinA treatment for CM improved HRQoL (as measured by the Migraine-Specific Quality of Life Questionnaire [MSQ]) and reduced headache days, with high physician and participant satisfaction. Reference Boudreau, Finkelstein and Graboski34 The objective of this analysis was to descriptively assess the impact of long-term treatment with onabotulinumtoxinA on HRU, work productivity, and acute medication use in participants from the PREDICT study.
Methods
Study Design
The design of the PREDICT study has been published previously. Reference Boudreau, Finkelstein and Graboski34 Briefly, PREDICT was a prospective, observational standard of care study conducted across 16 centers in Canada to assess long-term HRQoL in adults with CM receiving onabotulinumtoxinA treatment. Eligible participants were prescribed a 2-year regimen (up to 7 treatment cycles) of onabotulinumtoxinA administered according to the PREEMPT paradigm Reference Blumenfeld, Silberstein, Dodick, Aurora, Turkel and Binder35,Reference Blumenfeld, Silberstein, Dodick, Aurora, Brin and Binder36 every 12 weeks per the Canadian onabotulinumtoxinA product monograph (version July 7, 2014 37 ). The PREEMPT paradigm recommends 155 units (U) of onabotulinumtoxinA administered as 31 fixed-site, fixed-dose intramuscular injections across 7 specific head/neck muscle areas. If the participant reported a predominant pain location(s), the dosage could be adjusted with optional additional injections to one or both sides in up to 3 specific muscle groups (temporalis, occipitalis, and trapezius), for a maximum dose of 195 U onabotulinumtoxinA administered to 39 sites per treatment. Physicians who participated in the PREDICT study received a recommendation to follow the PREEMPT paradigm, though it was not enforced. PREDICT consisted of 7 study visits, including a screening visit (visit 1/baseline), onabotulinumtoxinA treatment 1 (visit 2), treatment 2 (visit 3), treatment 3 (visit 4), treatment 4 (visit 5), and treatment 7 (visit 6), and the final study visit (visit 7). When participants returned to the clinic for treatment 5 and treatment 6, no other study-related procedures were required. Study participants did not receive financial support for any treatment or treatment-related costs and accessed onabotulinumtoxinA through their usual means for obtaining prescription medications.
PREDICT was conducted following all relevant regulatory guidelines, including the International Conference on Harmonisation Guideline for Good Clinical Practice. Approval of the study protocol was obtained by each investigator from a properly constituted Research Ethics Board (REB) before initiating the study.
Participants
Eligible participants included adult men and women, ≥18 years of age with a diagnosis of CM (as defined by the ICHD-3, beta version 38 ) for whom treatment with onabotulinumtoxinA was deemed medically necessary by the participating physician independently from the study. All study participants were naïve to onabotulinumtoxinA treatment for CM at baseline. Participants were permitted to use other migraine-specific preventive medication(s) if the dose was stable and well-tolerated for at least 12 weeks prior to screening. Participants currently taking or planning to take opioid-containing products, barbiturates, or combination analgesics for acute headache treatment or a pain condition on >8 days during the baseline period were excluded. The full inclusion and exclusion criteria are provided in Supplemental Table 1. 37–Reference Beck, Steer, Ball and Ranieri39 All participants were provided with and signed an REB-approved informed consent form prior to study enrollment.
Health Resource Utilization
Participants completed an HRU questionnaire at visit 1/screening, visit 3/treatment 2, visit 5/treatment 4, and visit 7/final visit. The HRU questionnaire consisted of four questions addressing HCP evaluation/treatment of headache, ER or urgent care (UC) clinic visits for headache treatment, hospital admissions and overnight stays for headache, and headache-related diagnostic testing within the past 6 months (Supplemental Table 2). An overnight hospital stay was defined as being in a hospital bed at midnight following admission.
Work Productivity
To examine the impact of onabotulinumtoxinA treatment on work productivity, participants completed the Work Productivity and Activity Impairment Questionnaire: Specific Health Problem V2.0 (WPAI:SHP) Reference Reilly, Zbrozek and Dukes40 at visit 1/screening, visit 5/treatment 4, and visit 7/final visit. The WPAI:SHP is a 6-item questionnaire that assesses the effect of CM on the ability to work and perform regular daily activities over the past 7 days. The questions ask participants to report the number of hours missed from work due to headache as well as how much headache affected work productivity (rated on an 11-point scale). The questionnaire also asks how much headache affected the ability to do regular non-work-related daily activities (rated on an 11-point scale).
Acute Medication Use
The use of medication for the acute treatment of migraine attacks between study visits was collected in a headache diary, which was completed daily by participants throughout the study. The headache diary was distributed at visits 1 through 6 and collected at visits 2 through 7. The baseline analysis window for acute medication use was the first 28 days following visit 1/screening preceding the first onabotulinumtoxinA injection treatment at visit 2/treatment 1 (Day 1). For visit 2/treatment 1, visit 3/treatment 2, visit 4/treatment 3, and visit 5/treatment 4, the analysis window began on Day 2 (the day after onabotulinumtoxinA injection) and ended on Day 84 relative to Day 1 for each treatment.
Safety
Treatment-emergent adverse event (TEAE) data were tabulated by system organ class (SOC) and preferred term (PT). TEAEs with a reasonable relationship to onabotulinumtoxinA injections were summarized by overall counts and percentages in each SOC by PT and categorized by maximum severity (mild, moderate, severe) within each SOC. All TEAEs associated with possible distant spread of toxin were tabulated by SOC and PT and listed by participant.
Statistical Methods
This is a prespecified secondary analysis of the PREDICT study data. The study was observational only; no formal hypothesis was tested, and no formal sample size calculations were performed. Data from all clinical sites were combined for analysis.
The analysis population included all study participants who received at least one treatment with onabotulinumtoxinA. HRU and work productivity results were summarized and are presented descriptively by study visit. Continuous variables were summarized descriptively by the number of observations, the number of missing observations, mean, standard deviation (SD), median, and range (minimum/maximum). Categorical variables were summarized descriptively as frequency counts and percentages. The mean number of acute medication days per 28-day period at baseline (visit 1/screening) and treatments 1 through 7 were calculated using a prorated approach to standardize the original count to a 28-day count for the observed data as follows: mean number of acute medication days per 28-day period = (number of symptomatic medication days) * (28/number of days available). Change from baseline in the mean number of acute medication days at treatments 1 through 7 was summarized descriptively and analyzed using a two-sided Wilcoxon matched-pairs signed-rank test, performed at the two-sided 0.05 significance level. Only participants who recorded ≥15 and ≤28 days of headache diary data in the baseline window and who had ≥45 and ≤83 days of headache diary data available in the Day 2 to Day 84 analysis window for treatments 1 through 4 were included in this analysis. These timeframes were chosen to standardize the assessment period to 28 days, and data collected outside of these timeframes were not included in the analyses. Missing data were not imputed. P-values were not adjusted for multiple comparisons. Data from all clinical sites were combined for analysis.
Results
Participant Characteristics
The PREDICT study enrolled a total of 197 participants, 184 (93.4%) of whom received at least one treatment with onabotulinumtoxinA and were included in the analysis population (Figure 1). Overall, 123/197 (62.4%) participants completed the 2-year study (7 treatment cycles) and 74/197 (37.6%) participants discontinued the study. Eighty-two percent of participants who discontinued the study completed a withdrawal questionnaire that provides additional details about the reason(s) for discontinuation. According to the withdrawal questionnaire, the most common reasons for discontinuation were “treatment did not work” (23/184; 12.5%) and “cost of the injection treatment” (5/184; 2.7%).

Figure 1: PREDICT study participant disposition. Diagram of PREDICT study participant disposition, with the number of participants who were excluded from the study and the number of participants that withdrew from the study shown. No data were missing. These discontinuation data were collected by the investigator. A withdrawal questionnaire that was completed by participants is reported separately. n or N=the number of participants.
Baseline participant demographic and clinical characteristics are presented in Table 1. PREDICT study participants were 44.8 years of age on average, predominantly female (156/184; 84.8%) and Caucasian (174/184; 94.6%), and reported a mean (SD) of 23.1 (5.7) headache days/month in the past 3 months. The majority of participants (78.8%) reported taking preventive medication(s) for CM in the past 2 years. Mean (SD) baseline acute medication(s) use for CM was 16.8 (9.0) days/month over the past 3 months.
Table 1: Baseline participant demographics, chronic migraine history, and medication use history†

CM=chronic migraine; n=number of participants; SD=standard deviation.
† Missing data include: 6 participants for age at diagnosis and age participant started having headaches, 2 participants for family history, and 1 participant for days/month of acute medication.
‡ The top 3 most frequently used medication types are shown; participants could report >1 preventive medication.
OnabotulinumtoxinA Treatment
Overall, the mean (SD) study enrollment was 17.9 (7.0) months. The mean (SD) duration between each of the first 4 onabotulinumtoxinA treatments was 13.2 (1.8) weeks and the mean (SD) total dose of onabotulinumtoxinA injected per treatment across all treatments was 171 (18) U (Supplemental Table 3). At onabotulinumtoxinA treatments 1–4, 98.1% (658/671) of participants received a mean total injection dose between 155 U and 200 U.
Health Resource Utilization
In PREDICT, all measures of HRU improved with onabotulinumtoxinA treatment for CM. At baseline, a total of 96.2% (177/184) of participants reported visiting an HCP for treatment or evaluation of their headache within the past 6 months. As presented in Table 2, this percentage decreased at onabotulinumtoxinA treatment 2 (87.9%, 152/173), treatment 4 (77.9%, 116/149), and at the final study visit (76.8%, 109/142). The most frequently visited HCPs reported across all time points were neurologists/headache specialists, primary care physicians, and pain specialists. At baseline, 73.4% (130/177) of participants reported visiting a neurologist/headache specialist, 68.9% (122/177) a primary care physician, and 20.9% (37/177) a pain specialist. At the final study visit, a similar percentage of participants reported visits to a neurologist/headache specialist (70.6%, 77/109) or pain specialist (21.1%, 23/109); however, the percentage of patients that reported a visit to their primary care physician decreased to 27.5% (30/109) (Table 2).
Table 2: Healthcare professional visits for headache treatment or evaluation within the past 6 months
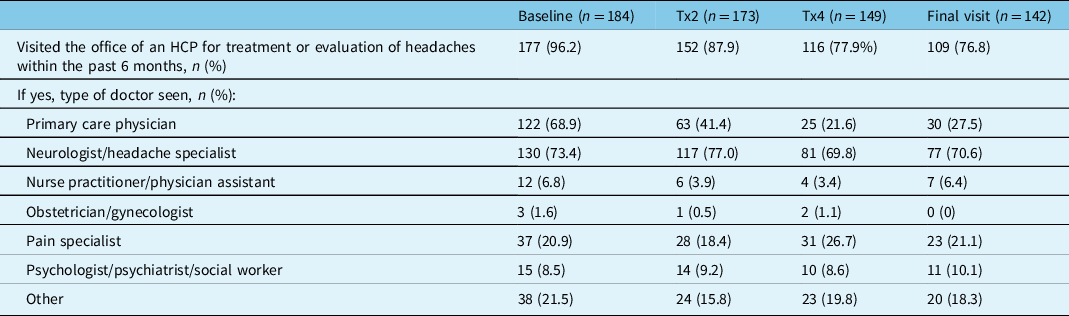
HCP=healthcare professional; n=number of participants; Tx=treatment.
The percentage of participants that reported visiting an ER or UC clinic within the past 6 months to receive treatment for their headache decreased from 17.9% (33/184) at baseline to 8.4% (12/143) at the final study visit (Figure 2A). There was also a consistent decrease in the mean number of ER or UC clinic visits reported at onabotulinumtoxinA treatments 2 and 4 and the final study visit (Figure 2B). The percentage of participants that reported a headache-related hospital admission in the past 6 months decreased from 3.8% (7/184) at baseline to 2.9% (5/172) at treatment 2, 1.4% (2/148) at treatment 4, and 1.4% (2/140) at the final study visit. The median number of hospital admissions and overnight hospital stays also decreased from baseline to the final visit (Table 3).
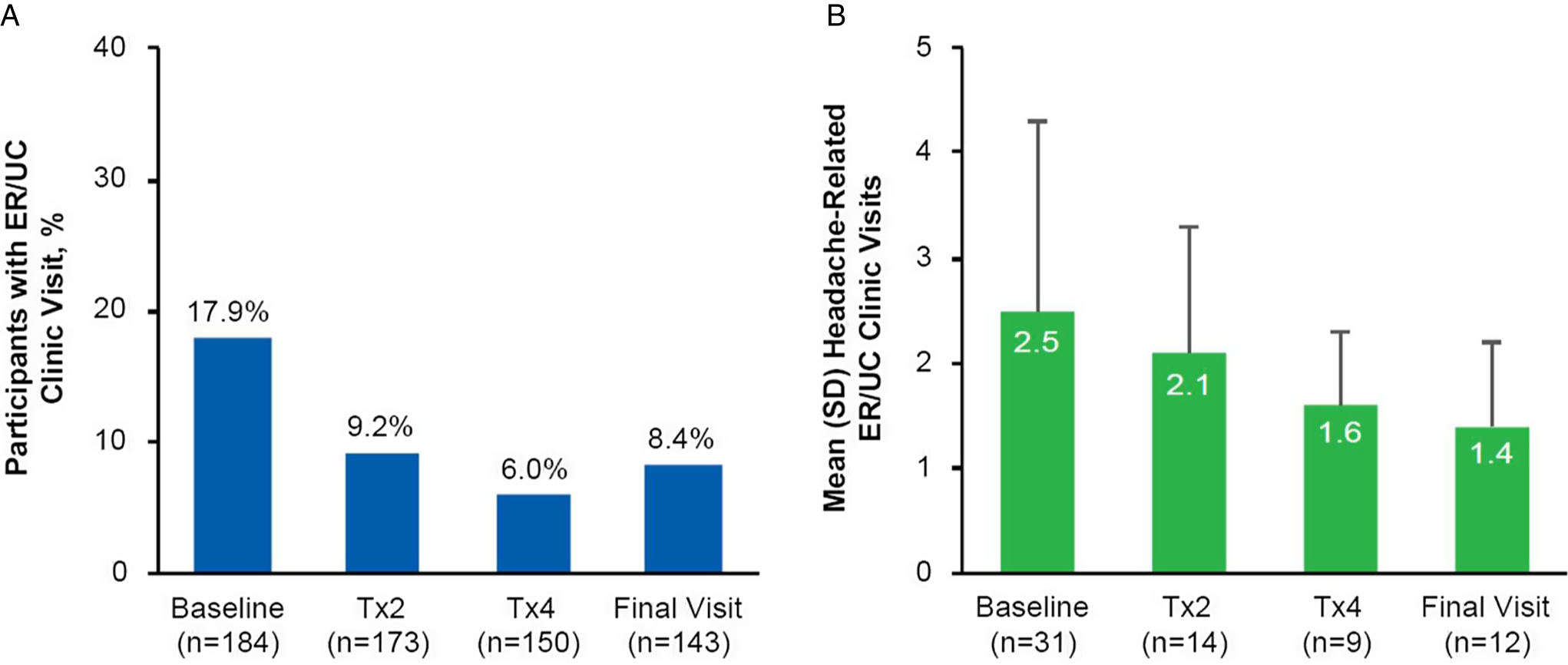
Figure 2: PREDICT health resource questionnaire: headache-related visits to an emergency room or urgent care clinic. (A) Percentage of participants at baseline (n=184), Tx2 (n=173), Tx4 (n=150), and the final visit (n=143) who reported visiting an ER or UC clinic to receive headache treatment within the past 6 months; (B) Mean (SD) number of headache-related visits to an ER or UC clinic within the past 6 months as reported by participants who reported ER or UC visits at baseline (n=31), Tx2 (n=14), Tx4 (n=9), and the final visit (n=12). Missing data include 11 participants at Tx2, 34 participants at Tx4, and 41 participants at the final visit. ER=emergency room; n=number of participants; Tx=treatment; UC=urgent care.
Table 3: Headache-related hospital admissions within the past 6 months
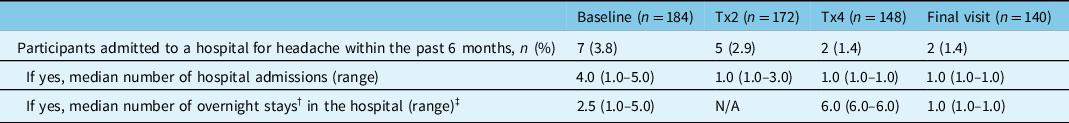
n=number of participants; N/A=not available; Tx=treatment.
† An overnight hospital stay was defined as being in a hospital bed at midnight.
‡ Missing data include: 1 participant at baseline, 5 participants at treatment 2, 1 participant at treatment 4, and 1 participant at final visit.
The percentage of participants who underwent headache-related diagnostic testing within the past 6 months decreased with onabotulinumtoxinA treatment. At baseline, 37.5% (69/184) of participants reported receiving headache-related diagnostic testing, which decreased to 16.4% (28/171) of participants at treatment 2, 9.3% (14/150) of participants at treatment 4, and 9.9% (14/142) at the final study visit (Table 4). The most common diagnostic tests reported across all time points were blood tests, magnetic resonance imaging, and computerized axial tomography.
Table 4: Headache-related diagnostic testing within the past 6 months
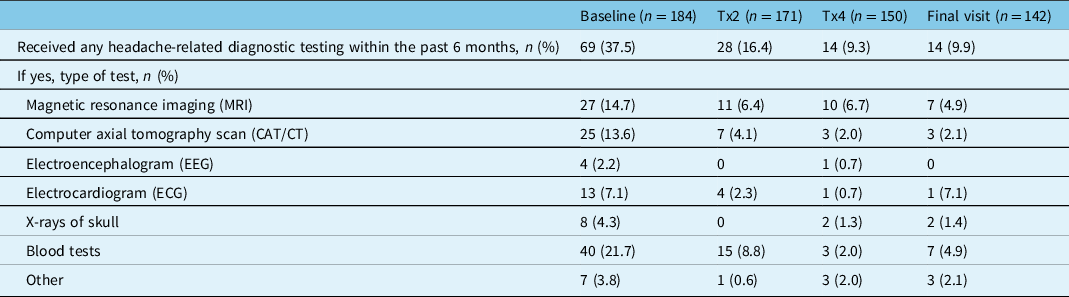
n=number of participants; Tx=treatment.
Work Productivity (WPAI-SHP)
The results of the WPAI:SHP questionnaire are presented in Table 5. The percentage of participants working for pay was similar across study time points with a range of 70.8%–73.4%. The number of hours missed from work in the past 7 days due to headache-related problems decreased with onabotulinumtoxinA treatment from a mean (SD) of 6.1 (9.7) hours at baseline to 2.9 (6.8) hours at visit 5/treatment 4 and 3.0 (6.8) hours at the final visit. Participants working for pay also reported a decrease in work hours missed due to non-headache-related reasons and an increase in the mean number of total working hours at visit 5/treatment 4 and the final visit compared to baseline. The effect of headache on work productivity decreased from a mean (SD) of 5.4 (2.1) at baseline to 3.9 (2.5) at visit 5/treatment 4 and 3.6 (2.4) at the final visit. A similar change was seen for the effect of headache on the ability to perform non-work-related daily activities, which decreased from a mean (SD) of 6.2 (2.1) at baseline to 4.2 (2.7) at visit 5/treatment 4 and 4.2 (2.8) at the final visit. As shown in Table 5, onabotulinumtoxinA treatment improved productivity while working and reduced the impact of headaches on regular daily activities other than work at a job in participants working for pay, as evidenced by lower scores.
Table 5: Work Productivity and Activity Impairment Questionnaire: Specific Health Problem V2.0 (WPAI-SHP)
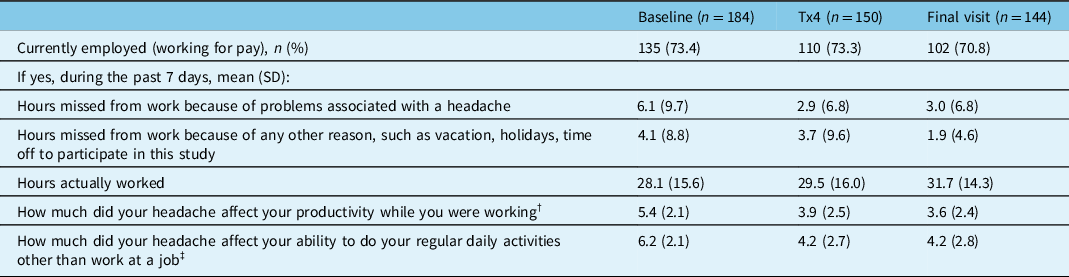
n=number of participants; SD=standard deviation; Tx=treatment.
† 11-point scale: 0 = headache had no effect on work, 10 = headache completely prevented me from working.
‡ 11-point scale: 0 = headache had no effect on daily activities, 10 = headache completely prevented me from daily activities.
Acute Medication Use
Data collected from the headache diary showed a decrease in the mean (SD) number of acute medication use days from baseline across onabotulinumtoxinA treatments 1, 2, 3, 4, and 7 with a range of 15.2 (7.6) days at baseline to 9.1 (6.5) days at treatment 7 (Figure 3A). As presented in Figure 3B, there was a statistically significant decrease (p < 0.05) in the mean paired change from baseline in the mean number of acute medication days at treatments 1, 2, 3, 4, and 7. The largest decrease from baseline in the mean number of acute medication days was observed at treatment 7, with a mean (SD) reduction of 6.0 (7.7) days per 28-day period.
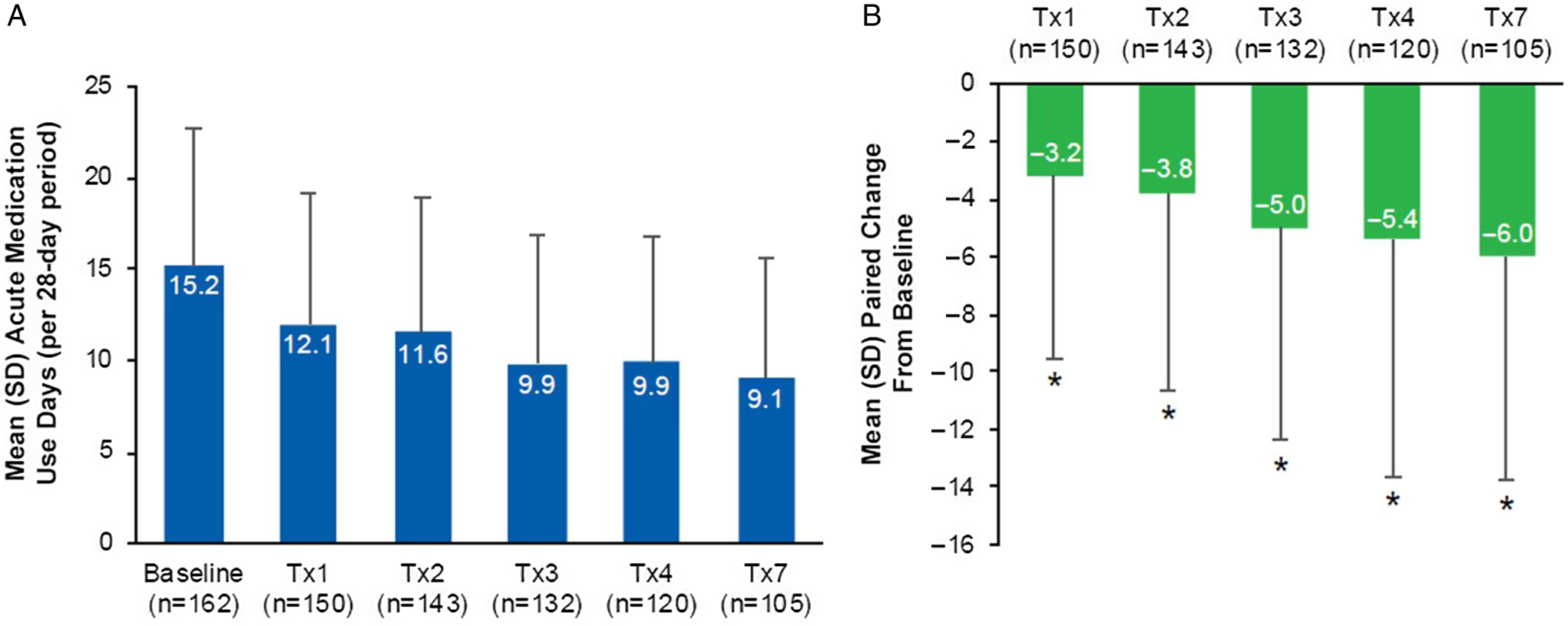
Figure 3: Acute medication use per 28-day period in the PREDICT study. (A) Mean (SD) acute medication use days for the treatment of migraine attacks per 28-day period at baseline (n=162), Tx1 (n=150), Tx2 (n=143), Tx3 (n=132), Tx4 (n=120), and Tx7 (n=105); (B) Mean (SD) paired change from baseline in acute medication use days per 28-day period at Tx1 (n=150), Tx2 (n=143), Tx3 (n=132), Tx4 (n=120), and Tx7 (n=105). *Indicates p<0.001. Paired change within patient from baseline values derived as time point minus baseline. SD=standard deviation; Tx=treatment.
Safety
In the PREDICT study, 168 TEAEs were reported in 77 participants (77/184, 41.8%); 38 events in 22 participants (22/184, 12.0%) were considered treatment related (Supplemental Table 4). The most common treatment-related TEAE was eyelid ptosis. Six serious adverse events were reported in four participants (4/184, 2.2%), none of which were considered treatment related. These events were experienced by one participant each and were primarily nervous system disorders, which included central pain syndrome, idiopathic intracranial hypertension, seizure, and syncope. A single case of colon cancer was also reported. There was no evidence of distant spread of toxin. Overall, three participants (3/184, 1.6%) discontinued the study due to a TEAE. No new safety signals were identified.
Discussion
This analysis of secondary outcomes from the PREDICT study showed a positive impact of real-world long-term onabotulinumtoxinA treatment on HRU, work productivity, and acute medication use in a population of Canadian adults with CM. Our findings demonstrate that onabotulinumtoxinA reduced headache-related HCP visits, ER/UC clinic visits, hospital admissions, and diagnostic testing within 2 treatment cycles (∼6 months) and up to 2 years. Additionally, we observed a statistically significant decrease in acute medication use for CM between baseline and onabotulinumtoxinA treatments 1 through 7. In PREDICT participants working for pay, onabotulinumtoxinA treatment reduced the number of headache-related hours missed from work, increased the total number of hours worked, and improved work productivity and activity impairment. OnabotulinumtoxinA treatment was well tolerated with no new safety signals identified.
As a key driver of the economic burden due to direct costs associated with CM, HRU is an important indicator of effective migraine treatment. Consistent with the results of other real-world studies, Reference Rothrock, Bloudek, Houle, Andress-Rothrock and Varon29,Reference Hepp, Rosen, Gillard, Varon, Mathew and Dodick31–Reference Rothrock, Stark, Sommer and Blumenfeld33 onabotulinumtoxinA treatment for CM improved multiple measures of HRU in PREDICT. The percentage of participants who reported a headache-related visit to an HCP decreased with onabotulinumtoxinA treatment at each time point across the study. This was largely driven by the 41% decrease in the percentage of participants reporting primary care visits between baseline (122/177, 68.9%) and the final study visit (30/109, 27.5%). Inferences from this study related to the impact of onabotulinumtoxinA treatment on neurologist/headache specialist visits are limited by the nature of the HRU questionnaire, which did not specify whether onabotulinumtoxinA injectors should be included in the response. As subjects were seeing a neurologist or headache specialist for their onabotulinumtoxinA injections every 3 months, this is likely the reason why visits to neurologists or headache specialists were not reduced during the study. Overall, the reductions in the percentage of participants reporting headache-related HCP and ER/UC clinic visits, diagnostic testing, and hospital admissions in PREDICT underscore the treatment benefit and cost-effectiveness of onabotulinumtoxinA for CM in this population.
PREDICT is one of the first real-world observational studies to assess the impact of onabotulinumtoxinA on HRU beyond 1 year. In an open-label study in patients with CM who were refractory to ≥2 oral prophylactic medications, Rothrock et al. Reference Rothrock, Bloudek, Houle, Andress-Rothrock and Varon29 analyzed the impact of onabotulinumtoxinA treatment on the frequency and cost of migraine-associated HRU, including ER visits, UC visits, and hospitalizations 6 months before and after initial treatment. In this study, there were 55% fewer ER visits, 59% fewer UC visits, and 57% fewer hospitalizations during the 6-month treatment period (2 treatment cycles) than the 6 months before the initial treatment. The treatment-related cost analysis showed that onabotulinumtoxinA treatment yielded an average savings of $1219.33/participant, which offset the total estimated cost for 6 months of onabotulinumtoxinA injections by 49.7%. Reference Rothrock, Bloudek, Houle, Andress-Rothrock and Varon29 Using a large, US-based, healthcare claims database, Hepp et al. Reference Hepp, Rosen, Gillard, Varon, Mathew and Dodick31 evaluated ER visits and hospitalizations in adults with CM treated with onabotulinumtoxinA or oral migraine prophylactic medications. The proportion of individuals with headache-related ER visits or hospitalizations decreased at 6, 9, and 12 months after starting onabotulinumtoxinA treatment but increased after starting oral migraine prophylactic medications. Reference Hepp, Rosen, Gillard, Varon, Mathew and Dodick31 Recently, two long-term, real-world studies with designs similar to PREDICT examined the impact of onabotulinumtoxinA treatment on HRU in European (REPOSE) Reference Kollewe, Antonakakis, Kiszka, Sommer and Yu32 and US (COMPEL) Reference Rothrock, Stark, Sommer and Blumenfeld33 adult populations with CM. In the REPOSE study, Kollewe et al. Reference Kollewe, Antonakakis, Kiszka, Sommer and Yu32 observed reductions from baseline in HCP visits for any reason, emergency visits for any reason, and headache-related hospitalizations. In COMPEL, Reference Rothrock, Stark, Sommer and Blumenfeld33 the frequency of headache-related visits to an HCP, headache-related visits to an ER/UC clinic, and overnight hospitalizations decreased significantly from baseline at all time points. As in PREDICT, Rothrock et al. also observed a decrease in the number of headache-related diagnostic tests reported by participants from weeks 24 to 96 of the COMPEL study. Reference Rothrock, Stark, Sommer and Blumenfeld33 The consistency of region-specific data on the impact of onabotulinumtoxinA treatment on HRU is important for understanding the scope of onabotulinumtoxinA treatment benefit for CM in real-world clinical settings across different healthcare systems and patient populations.
In addition to HRU, the impact of CM on work productivity creates a substantial economic burden for individuals with CM, their families, and employers. CM is associated with both productivity loss resulting from reduced performance at work (presenteeism) and absence from work due to disability (absenteeism). Reference Baigi and Stewart16,Reference Serrano, Manack, Reed, Buse, Varon and Lipton18 In the internet-based Chronic Migraine Epidemiology and Outcomes (CaMEO) study, over half of the respondents indicated that migraine negatively affected ≥1 career area (654/1119; 58.4%) and caused them to worry about long-term financial security (641/1116; 57.4%). Reference Buse, Fanning and Reed9 With over 70% of the PREDICT study population working for pay, the results strengthen the evidence base demonstrating the efficacy of onabotulinumtoxinA treatment to increase total working hours, reduce headache-related hours missed from work, and reduce the impact of migraine on work productivity and regular daily activity. Recently, the FORWARD study, Reference Blumenfeld, Patel, Adams and Rothrock41 a multicenter, randomized study conducted in the USA, compared onabotulinumtoxinA treatment for CM with topiramate on work productivity as measured by WPAI-SHP scores. OnabotulinumtoxinA improved the work productivity loss (p = 0.024) and activity impairment (p < 0.001) domains to a significantly greater extent than topiramate. At 36 weeks, onabotulinumtoxinA treatment was also associated with a reduction in mean absenteeism and presenteeism scores from baseline. Reference Blumenfeld, Patel, Adams and Rothrock41 In an Australian study by Stark et al. Reference Stark, Stark and Limberg27 , onabotulinumtoxinA naïve patients with inadequately controlled CM reported significant improvements from baseline in missed work or study days after 2 treatment cycles and through the final visit (all p < 0.0001). Collectively, these findings suggest that treatment with onabotulinumtoxinA can improve work productivity and ease the individual and societal economic burden imposed by CM.
Data from CHORD suggested that 21% of individuals with CM in the registry suffered from MO, caused by the overuse of medication(s) intended for the acute treatment of migraine. Reference Jelinski, Becker and Christie19 This condition can exacerbate the disability and diminished HRQoL experienced by individuals with CM and further impact HRU and work productivity. The initiation of effective preventive treatment is recommended to reduce the frequency of acute medication use in those with CM to prevent MO in those at risk. Reference Diener, Holle, Solbach and Gaul42 In PREDICT, CM treatment with onabotulinumtoxinA reduced mean acute medication use days per 28-day period by 39% from baseline to treatment 7 with a statistically significant mean paired change between baseline and all time points. These decreases closely correspond with the 32% reduction in mean monthly headache days and 46% reduction in mean moderate/severe monthly headache days from baseline to the final visit observed in PREDICT. Reference Boudreau43 Though PREDICT did not collect data to identify MO in participants, the impact of onabotulinumtoxinA treatment on acute medication use is significant and consistent with results from previous studies of individuals with CM both with and without MO. Reference Negro, Curto, Lionetto, Crialesi and Martelletti25,Reference Silberstein, Blumenfeld and Cady26,Reference Ahmed, Zafar, Buture and Khalil44,Reference Negro, Curto, Lionetto and Martelletti45 For instance, a prospective, 2-year analysis of patients with CM and MO by Negro et al. Reference Negro, Curto, Lionetto, Crialesi and Martelletti25,Reference Negro, Curto, Lionetto and Martelletti45 showed that the initiation of treatment with 155 U or 195 U onabotulinumtoxinA according to the PREEMPT paradigm resulted in a significant reduction in acute medication intake days compared with baseline. Similar to PREDICT, the reduction was significant after the first injection and gradually increased during the 2-year treatment period. Reduction of acute medication use can lower the risk of developing MO and alleviate symptoms in those who already have MO, thereby attenuating both the physical and financial burdens suffered by individuals with CM.
When interpreting the results of this analysis, certain limitations inherent to observational studies should be noted. In observational, standard-of-care studies, there is no placebo arm to serve as a comparator for treatment outcomes. Due to the observational nature of the study, outcome measures were reported as collected during individual patient visits, the timings of which may have varied. Additionally, the use of self-reported data is subject to recall bias and information bias. The results of long-term studies may also be impacted by loss to follow-up and the high number of participants who discontinued treatment and had missing data on some outcomes (e.g., HRU), with the population remaining at later time points consisting mostly of treatment responders. In other words, the benefits that we noted from onabotulinumtoxinA therapy do not necessarily apply to all patients who are administered this treatment but rather to those who continue treatment. Individuals with CM who continue treatment likely do so primarily because they perceive a benefit. It was recommended that physicians follow PREEMPT guidelines; however, this portion of the protocol was not enforced and, therefore, the results may not be generalizable to the wider population. This aspect of the study design could have resulted in the variability observed in the time between onabotulinumtoxinA injections and the broad range of doses administered. Notably, these variations were attributable to a small number of outliers. Although the Headache Impact Test-6 and Migraine Disability Assessment Test would have been valuable additions to this data set to help assess disability, functioning, and quality of life, they were not included in the original PREDICT protocol and were therefore not assessed. Additionally, the WPAI-SHP has not been validated in migraine, which is a limitation of all studies using this measure. It is also possible that reductions in acute MO and/or changes in the use of oral preventive medications may have been a confounding factor that could have influenced the results of the study. The reduction in primary care, UC, and emergency department visits could also possibly be due to the administration of follow-up onabotulinumtoxinA injections by a neurologist, who may have altered the treatment plan based on treatment response by educating patients on proper rescue medication use. Additionally, performing baseline headache-related diagnostic testing 6 months prior to initiating onabotulinumtoxinA treatment could have resulted in non-headache specialist providers not repeating imaging tests such as magnetic resonance imaging, computer axial tomography scan, and electroencephalogram, thereby reducing HRU. Lastly, as the objectives of this study were descriptive in nature, there were no statistical analyses performed for HRU and work productivity outcomes. However, the results from PREDICT are consistent with previous findings published in this area. Despite these limitations, as a standard of care study, data from PREDICT are generalizable to real-world clinical settings. The demographic and clinical characteristics of the PREDICT study population are consistent with those from previous studies and reflect the typically reported CM migraine population in Canada and other countries around the world. The positive impact of onabotulinumtoxinA treatment on HRU, work productivity, and acute medication use observed in the PREDICT study supports the broad real-world treatment benefit of onabotulinumtoxinA for CM.
Conclusions
Real-world evidence from the PREDICT study demonstrates that CM treatment with onabotulinumtoxinA in the Canadian population is associated with improvements in HRQoL and reductions in HRU, including headache-related visits to an HCP, ER and UC clinic visits, hospital admissions, and diagnostic testing. OnabotulinumtoxinA treatment also improved participant-reported workplace productivity and reduced daily activity impairment and acute medication use. These data support previous reports of long-term benefits associated with the use of onabotulinumtoxinA for the treatment of CM in clinical practice.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/cjn.2022.43.
Acknowledgements
The authors sincerely thank the participants and investigators who participated in this study. Medical writing was provided to the authors by Kristin M. Hirahatake, PhD of AbbVie. Editorial assistance was provided by Peloton Advantage, LLC, an OPEN Health company, Parsippany, NJ, and was funded by AbbVie. Statistical analyses were done by ClinWest. All authors met the ICMJE authorship criteria. Neither honoraria nor any other form of compensation was provided for authorship.
Funding
This study was sponsored by Allergan (prior to its acquisition by AbbVie), Markham, Ontario, Canada. Allergan, now AbbVie, participated in the analysis and interpretation of data; and writing, reviewing, and approval of the final version. No honoraria or payments were made for authorship.
Disclosures
Dr. WJB received compensation for consulting and/or honoraria from Allergan (now AbbVie), Aralez, Eli Lilly & Co., Lundbeck, Novartis, and Weber and Weber. Dr. GB received compensation for consulting, honoraria, and/or research support from Allergan (now AbbVie), Lilly, Novartis, and Teva. Dr. IF received compensation for consulting and/or honoraria from Allergan (now AbbVie), Aralez, Lilly, Novartis, Nuvo, and Teva. Dr. CG received compensation for consulting and/or honoraria from Allergan (now AbbVie), Aralez, Amgen/Novartis, and Lilly. Dr. MO received compensation for consulting and/or honoraria from Cannimed and Spectrum Canada. Dr. SC received compensation for consulting and/or honoraria from Allergan (now AbbVie), Lilly, Novartis, and Teva, and received research support from Novartis. Dr. KS, Mr. MB, and Dr. GD are employees of AbbVie. Dr. KS and Dr. GD own stock or stock options in AbbVie.
Statement of Authorship
WJB analyzed and interpreted the data and revised the manuscript for intellectual content. GB participated as a study investigator, provided critical review, and approved the final draft. IF participated as a study investigator, provided critical review, and approved the final draft. CG participated as a study investigator, provided critical review, and approved the final draft. MO participated as a study investigator, provided critical review, and approved the final draft. SC provided critical review and approved the final draft. KS completed data analysis and interpretation, was involved in manuscript preparation, provided critical review and revisions, and approved the final draft. MB completed data analysis and interpretation, was involved in manuscript preparation, provided critical review and revisions, and approved the final draft. GD completed data analysis and interpretation, was involved in manuscript preparation, provided critical review and revisions, and approved the final draft. All authors reviewed and approved the final manuscript for publication.










