Amyotrophic lateral sclerosis (ALS) is a progressive neurological disease resulting from the death of upper and lower motor neurons in the motor cortex, brain stem and spinal cord. This results in rapidly progressive muscle weakness, atrophy, and spasticity. Bulbar and respiratory muscle involvement can lead to dysphagia and dyspnea. In addition to the management of neuromuscular symptoms, nutritional and respiratory management are important aspects in the treatment of ALS.
Weight loss and dysphagia are frequent features of ALS and influence prognosis. The etiology of weight loss is multi-factorial. Upon initial diagnosis, patients with ALS are generally lean with a normal or low body-mass index (BMI).Reference Vaisman, Lusaus and Nefussy 1 Patients then typically become malnourished and lose body fat as the disease progresses resulting in a further reduction in BMI.Reference Kasarskis, Berryman, Vanderleest, Schneider and McClain 2 - Reference Mazzini, Corra, Zaccala, Mora, Del Piano and Galante 4
The reason for progressive weight loss is two-fold; a decrease in energy intake is coupled with an increase in energy expenditure. Cranial bulbar muscle weakness producing dysphagia increases the risk for insufficient caloric intake; patients often eat more slowly and become fatigued during meals.Reference Desport, Preux, Truong, Vallat, Sautereau and Couratier 5 Patients with ALS have a high basal metabolism rate contributing to weight loss.Reference Desport, Preux and Magy 6 - Reference Desport, Torny, Lacoste, Preux and Couratier 7 The mechanism leading to hypermetabolism is currently unknown, but has been demonstrated in both the sporadic and familial forms of ALS.Reference Desport, Preux and Magy 6 - Reference Funalot, Desport, Sturtz, Camu and Couratier 8 A loss of body mass and malnutrition are associated with faster progression of the disease and are independent prognostic factors.Reference Desport, Preux, Truong, Vallat, Sautereau and Couratier 5 , Reference Stambler, Charatan and Cedarbaum 9 - Reference Marin, Desport and Kajeu 13 Of additional concern, are the safety issues of aspiration and choking that result from dysphagia.
Nutritional interventions are integral to the management of ALS. At the onset of dysphagia the initial steps involve counseling by dieticians, modification of food and fluid consistency, prescription of high-protein and high-caloric supplements, and education of the patient on feeding and swallowing techniques. However, if significant caloric reduction or aspiration risk develops, a gastrostomy feeding tube is often introduced. Both the American Academy of Neurology (AAN) and the European Federation of Neurological Sciences (EFNS) have published guidelines recommending the placement of percutaneous endoscopic gastrostomy (PEG) in patients with ALS in order to supplement nutrition. This recommendation was based on evidence that nutritional supplementation using PEG was helpful for stabilizing weight loss.Reference Miller, Jackson and Kasarskis 14 - Reference Katzberg and Benatar 16 The prevention in weight-loss provided by PEG likely translates to a survival benefit but there is not enough data to quantify to what extent this occurs.Reference Katzberg and Benatar 16
In addition to general procedural risks for PEG, such as wound infection, bleeding and ulceration, there are risks specific to the ALS population. Patients with respiratory muscle impairment undergoing sedation may have aspiration with PEG insertion.Reference Allen, Chen and Ajroud-Driss 17 Diaphragmatic weakness causes a “high-riding” stomach, in which the stomach lies under the ribs, which can increase the difficulty of tube insertion.Reference Shaw, Ampong, Rio, McClure, Leigh and Sidhu 18 Furthermore, ALS is known to be a rapidly progressing disease. Thus the timing and the method of insertion are important considerations when using gastrostomy feeding in the management of ALS. However, both these topics are under-studied. As a result, the AAN and EFNS have each stated that there is insufficient evidence to support or refute specific timing of PEG insertion in patients with ALS. That said, the AAN practice parameter does suggest consideration of dysphagia, weight loss and respiratory function, measured as forced vital capacity (FVC), in the decision making process.
Given the lack of evidence to rigorously guide decisions and the presence of international guidelines, we wished to determine how Canadian ALS clinics make decisions regarding the timing and placement of gastrostomy feeding tubes in patients with ALS.
Methods
Through email and paper correspondence we asked the medical directors of Canadian ALS clinics about their approach to gastrostomy feeding in patients with ALS. This information was also requested in the newsletter of the Canadian ALS Research Network (CALS). We asked two questions. Does the clinic have written centre-specific protocols to guide the timing of PEG placement in ALS patients? If a protocol existed we requested that they forward us any relevant documents. For centres that did not have written protocols we asked what steps are taken to determine candidacy and timing for placement of gastrostomy feeding tubes in lieu of formal guidelines. The responses were aggregated in order to compare and contrast the practices across clinic sites.
Results
A total of seventeen Canadian ALS clinics were contacted and ten centres provided a response. The results of the survey are summarized in Table 1. The open-ended questions led to variable amounts of detail in individual responses. One centre had centre specific written guidelines whereas the other nine did not. One of the ten responding centres was a francophone clinic. The remaining nine clinics were English speaking. All of the clinics were hospital affiliated. Nine of the ten responses commented on key decision-making criteria they consider in the decision to insert gastrostomy feeding tubes.
Most centres used a decline in respiratory function, dysphagia, weight loss or some combination of all three. Six clinics explicitly stated that they measure and consider a dropping FVC, a marker of respiratory decline, as a factor prompting the decision for feeding tube insertion. The FVC chosen ranged from >70% (with rapid decline) to <30% in special cases. One clinic reported inserting feeding tubes after tracheotomy and ventilation but stated this is a rare occurrence. Most recommend gastrostomy feeding at around FVC 50-60% of predicted. Swallowing impairment was reported as an important factor in decision-making by seven clinics but there was variation in how this was applied. Some clinics considered the number of aspiration events, others monitored prolonged mealtimes and patient and/or family concerns about swallowing. Two centres report regular use of swallowing assessments, either modified barium swallow or fiberoptic endoscopic evaluation of swallowing (FEES) to determine the extent of dysphagia and used this in their clinical decision making process. Five clinics reported weight loss in conjunction with, or independent of, other clinical factors could result in a referral for a feeding tube. Three centres used a quantifiable amount (>10% weight loss or <18.5 kg/m2 BMI in one centre and >10% weight loss in two centres) as triggering a need for gastrostomy feeding. Two others stated weight loss was considered but done through subjective assessment such as patient report. Two centres explicitly stated that they follow published guidelines for management of gastrostomy feeding in ALS.
Psychological readiness to have a tube placed was commented on by three sites as significantly influencing the recommendation for tube placement. Some centres note that discussions regarding future gastrostomy feeding are brought up early in the course of the disease to prepare the patient for future decision-making. Three sites reported that they frequently insert tubes well before oral feeding is impacted – sometimes more than a year in advance. This was generally due to reducing respiratory function in the absence of swallowing impairment. One clinic emphasized they recommended advanced health care directives to their patients that specifically include tube feeding decisions.
The method of feeding tube insertion varied considerably from centre to centre. Three centres report using PEG, three centres radiologically inserted gastrostomy (RIG) and four did not comment. One centre noted that the expertise for RIG was not available, hence all procedures are PEG. One centre requires admission to hospital for the procedure, but that was not a general requirement for the centres that reported.
Table 1 Canadian ALS clinic survey responses identifying which clinical factors prompt a referral for insertion of a feeding tube.
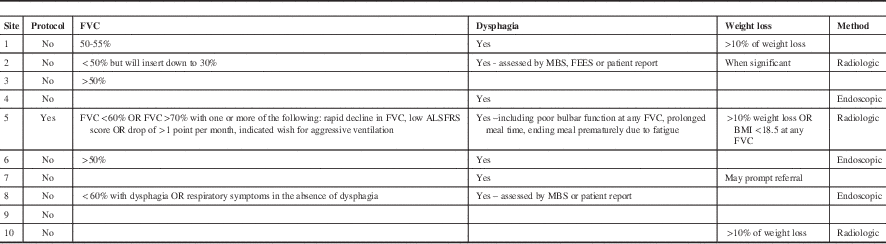
Notes: Clinic names have been removed. A blank entry denotes the respondent did not comment on this topic. FVC: Forced vital capacity. BMI: Body mass index kg/m2. ALSFRS: ALS Functional Rating Scale. MBS: Modified barium swallow. FEES: Fiberoptic endoscopic evaluation of swallowing.
Discussion
Weight loss and aspiration risks are concerns that can be adequately managed by gastrostomy feeding. The timing and criteria needed to insert a feeding tube is less clear. In lieu of good evidence there exists substantial variation in practice amongst Canadian ALS clinics.
Respiratory impairment, measured as FVC, was widely cited as prompting tube insertion. PEG placement requires mild sedation, which is thought to be hazardous in patients with compromised respiratory status. Currently the AAN practice parameter recommends PEG placement when FVC is greater than 50% predicted if dysphagia and weight loss are present. The parameter suggests caution in PEG insertion once the FVC is between 30-50%. Finally, it deems less than 30% predicted to be high risk.Reference Miller, Jackson and Kasarskis 14 With less than 30% predicted, the parameter recommends palliative intravenous hydration and nasogastric feeding to supplement any tolerated oral intake.
Certainly the relationship between respiratory compromise and PEG insertion has received attention in the literature. The benchmark FVC values set by the AAN are supported by studies that have recommended for optimal safety and efficacy that PEG be placed before the FVC falls to 50% of predicted due to increased rate of decline after this point.Reference Kasarskis, Scarlata and Hill 19 - Reference Andersen, Borasio and Dengler 20 Likewise, studies have reported that longer survival was associated with higher FVC at time of PEG insertion.Reference Chio, Finocchiaro, Meineri, Bottacchi and Schiffer 21 - Reference Desport, Preux, Truong, Courat, Vallat and Couratier 22 More recent literature complicates the picture, suggesting that PEG can be inserted at lower FVCs without impacting survival.Reference Gregory, Siderowf, Golaszewski and McCluskey 23 - Reference Dorst, Dupuis and Petri 26 Finally, assisting respiration during insertion, through non-invasive ventilator aids, can improve insertion outcomes in patients with severe respiratory muscle impairment.Reference Boitano, Jordan and Benditt 27 - Reference Sancho, Servera and Chiner 29 In our study six of the ten clinics reported using FVC to determine placement. Most felt comfortable placing PEG when FVC had fallen to 50-60% predicted. However, at least one clinic will perform gastrostomy tube insertion down to FVC 30% predicted. There were also differences in the likelihood that clinics would place feeding tubes well above the AAN benchmark of FVC 50%. Clearly respiratory involvement must be considered in the timing and placement of PEG but more research is needed to delineate the relationship.
Radiologically inserted gastrostomy (RIG) does not require sedation and can be an alternative to PEG when respiratory function is severely impaired. Studies that have compared outcomes of radiologic placed tubes with endoscopic placement are contradictive. Some studies have found radiologic placement to be more efficacious and better tolerated,Reference Thornton, Fotheringham, Alexander, Hardiman, McGrath and Lee 30 - Reference Blondet, Lebigot and Nicholas 31 whereas, other studies found no significant difference in efficacy.Reference Katzberg and Benatar 16 , Reference Desport, Mabrouk, Bouillet, Perna, Preux and Couratier 32 - 33 While growing in popularity, RIG is not as widely available and therefore PEG is used more often. Only three of our ten responding clinics reported using RIG for their ALS population. One centre had used RIG in the past but discontinued this practice after “significant negative experiences.” Regardless of the ambiguity in the literature with respect to RIG and PEG, consensus is that both are more efficacious than naso-gastric insertion in terms of survival and complication rate.Reference Scott and Austin 34 - Reference Gomes, Andriolo and Bennett 35 Despite higher rates of ulceration and discomfort, naso-gastric tubes can be used in the short term or when PEG/RIG are contraindicated.Reference Norton, Homer-Ward, Donnelly, Long and Holmes 36 - Reference Heffernan, Jenkinson and Holmes 37
Other relevant criteria that have been identified in the literature as likely to play an important in feeding tube placement include an unintentional and accelerated weight loss (often described as a loss of >10%), dysphagia, low BMI (generally less than 18 kg/m2) and failure of longitudinal nutritional assessments.Reference Heffernan, Jenkinson and Holmes 37 Both weight status and dysphagia were identified in our survey as being considered by clinics in the decision making process. It is intuitive that both would play a role in the decision to supplement oral feeding. These criteria, like respiratory benchmarks, require further research to understand what place they should take in the decision regarding gastrostomy feeding. Indicators of nutritional deficiency variably applied were identified as key elements of the evaluation of need for gastrostomy feeding in half of our reporting clinics, potentially reflecting uncertainty in how to integrate this information into the decision making process.
Though ALS clinics in Canada are known to be inter-disciplinary, our study did not ask whether clinics have dedicated gastroenterologists, dieticians, or speech language pathologists available during the decision making process. This is of relevance as studies have suggested inclusion of inter-disciplinary nutrition support teams results in higher rates of insertion.Reference Zhang, Sanders and Fraser 38 Once the decision is made to insert a feeding tube, dieticians and speech language pathologists help guide the choice of formula feed and infusion rate of feeds. This is another area of decision-making uncertainty as there are no ALS specific feeds or guidelines for artificial nutrition in ALS. Initial studies suggest that a high-carbohydrate hypercaloric enteral formula is ideal, tolerable, and safe but assessing the efficacy of specific feeds on disease progression is an area in need of further research.Reference Wills, Hubbard, Macklin, Glass, Tandan and Simpson 39
Differences between clinic sites and the lack of written centre-specific protocols has the potential to result in discrepancies in the management of ALS based on geographical location and/or time of presentation. Paramount in the effort to resolving these divergent care plans is the need for quality evidence-based guidelines. In order for such guidelines to be drafted there exists a need for more rigorous and ALS-specific research studies that examine the timing and insertion criteria for the placement of PEG.
This study used open-ended questions directed to medical directors to obtain information regarding practice in Canadian ALS clinics. A weakness of the study is a lack of data regarding actual practice, which might be divergent from the reported practice principles. The responses may not reflect which factors are deemed important in the nutritional management of all patients managed in the responding clinics. We did not ask for a hierarchical list of deciding factors in all patients and it is possible all clinics use some or all of the identified factors in different patient circumstances. A study analyzing clinic data on the key clinical and laboratory indicators that trigger the recommendation of gastrostomy feeding would most accurately describe the practice in Canadian ALS clinics.
Based on published guidelines and clinical practice highlighted in this report, patients with ALS should have an evaluation of their nutritional status soon after diagnosis and there should be reevaluation at each clinic visit.Reference Miller, Jackson and Kasarskis 14 The key parameters to assess include symptoms of dysphagia, BMI, and FVC. Patients should be provided with information regarding gastrostomy feeding early in their care. Patients with symptoms or laboratory evidence of dysphagia should be considered for gastrostomy. Likewise, patients with significant weight loss should also be considered for gastrostomy. Patients with failing respiration should be counseled to consider gastrostomy placement before respiratory function becomes too poor to safely insert a tube. If respiratory function is already poor, such as an FVC <50% when the decision to place a gastrostomy tube is made, then non-invasive ventilation around the time of insertion and the alternative of RIG should be considered.
In addition to more quantitative research into PEG criteria, there is a role for qualitative patient perspectives. To date, there is little published evidence regarding quality of life with respect to PEG insertion in patients with ALS. Preliminary research suggests that a feeding tube does give the patient a greater sense of control but may lead to increased anxiety.Reference O’Farrell, Strong, Zou and Rowe 40 This might be of particular relevance, as there seems to be a trend to recommend PEG earlier than required to avoid potential complications of late-placement. More quality of life studies are needed. Our study focused on the medical factors influencing the decision- making process for a referral for a feeding tube. For placement to take place, it requires a separate decision-making process on the part of the patient. Growing literature suggests that factors involved in the patient’s decision are even more complex and multi-faceted.Reference Martin, Landau and Janssen 41 , Reference Stavroulakis, Baird, Baxter, Walsh, Shaw and McDermott 42 Such decisions may benefit from an individualized approach to referral rather than an algorithmic approach.Reference Greenaway, Martin and Lawrence 43 The patient experience before, during and after insertion of a gastrostomy tube is an essential component of developing guidelines for what is essentially a palliative procedure. Information from patients about perceived nutritional needs; desires regarding timing of tube placement; benefits and difficulties noted after tube placement would all be important for development of effective guidelines. These questions may be answered by using qualitative research techniques.
Disclosures
Timothy Benstead, Caitlin Jackson-Tarlton, and Desmond Leddin do not have anything to disclose.



