Parkinson’s disease (PD) is diagnosed clinically by its classic triad of rest tremor, rigidity, and bradykinesia. These motor symptoms are the hallmark of the disease, but recent studies have highlighted the less-understood nonmotor symptoms (NMS) that may in part be related to pathology outside of the substantia nigra.Reference Chaudhuri, Healy and Schapira 1 These symptoms include smell disturbance, sleep disturbance (including rapid eye movement [REM] sleep behavior disorder), autonomic dysfunction, apathy, mood changes, and cognitive impairment. They may predate motor symptoms and are related to quality of life.Reference Chaudhuri, Healy and Schapira 1 Recent studies have shown that patients with specific NMS appear to have an increased likelihood of having or developing others. Baba et al.Reference Baba, Kikuchi and Hirayama 2 showed that over a 3-year period 10 of 24 (42%) PD patients with significant loss of smell developed dementia while none of the 20 (0%) PD controls without significant smell disturbance did. Similarly, Dujardin et al.Reference Dujardin, Sockeel, Delliaux, Destée and Defebvre 3 studied PD patients with and without apathy and over an 18-month period found development of dementia in 8/20 (40%) and 1/20 (5%), respectively. A more recent paper by Anang et al.Reference Anang, Gagnon and Bertrand 4 showed an increased risk of dementia with the baseline presence of REM sleep behavior disorder (RBD), orthostatic hypotension (OH), color discrimination, and gait disorder. This was further validated using two additional prospective cohorts suggesting age, sex, RBD, OH, and mild cognitive impairment as being significantly associated with dementia.Reference Anang, Nomura, Romenets, Nakashima, Gagnon and Postuma 5 A recent review paperReference McDonald, Newton and Burn 6 concluded that there appears to be a link between OH and cognitive impairment in PD, but it is unclear if this link is associative or causative. These studies suggest that PD patients who present with NMS without cognitive issues have an added risk of developing dementia over time. We aimed to further understand the connection between various NMS and the development of cognitive impairment in our population. We hypothesized that the presence of NMS—especially autonomic, olfactory, and neuropsychiatric features—at presentation would predict the progression of cognitive impairment.
Ethics approval was obtained by the university’s human research ethics board from the biomedical department, and informed consent was obtained prior to enrollment into the study for all participants. We enrolled a total of 52 patients as part of a longitudinal study. This patient population is as described by Camicioli et al.Reference Camicioli, Sabino and Gee 7 Patients had a clinical diagnosis of PD without significant cognitive impairment that interfered with function at baseline and were followed over a 36-month period. As previously described,Reference Camicioli, Sabino and Gee 7 we conducted a standardized neurological exam as well as assessments for NMS at baseline, 18 months, and 36 months. For the purpose of our study, the NMS screen included: psychiatric (mood and other behavioral changes) using the Neuropsychiatric Inventory for depression (NPI–Depression) (www.npitest.net) and for apathy (NPI–Apathy); olfactory function via the Brief Smell Identification Test (BSIT) and patient report of smell loss; sleep disturbances via the Mayo Fluctuation Scale,Reference Ferman, Smith and Boeve 8 NPI–Sleep, and the modified Epworth Sleepiness Scale;Reference Hobson, Lang, Martin, Razmy, Rivest and Fleming 9 and dysautonomia via an examination of orthostatic vitals in the lying, sitting, and standing positions. Orthostatic hypotension (OH) was defined as a drop of at least 20 mmHg of systolic pressure or 10 mmHg of diastolic pressure from lying to sitting or standing position or sitting to standing position if a patient was unable to tolerate lying flat. One patient was considered as having OH due to having borderline orthostatic hypotension (systolic drop of 16 mmHg) from lying to sitting, but felt so symptomatic that he was unable to attempt a standing position. The study neurologist and research assistant completed separate patient and caregiver interviews as well as patient assessments.
Our dependent variable was cognitive decline, and, as in our previous study, cognitive decline was assessed using a combination of the Mini-Mental Status Examination (MMSE), the Frontal Assessment Battery (FAB), the Dementia Rating Scale (DRS), and caregiver-derived (CDR) scores.Reference Camicioli, Sabino and Gee 7 Patients were assigned binary results as either having or not having clear cognitive decline based on the above scores and the clinical impression of the research neurologist (taking into account all information, including patient assessment, cognitive scores, and caregiver report based on a semistructured interview).
Over the course of our study, four patients were not included in the final analysis: two were excluded due to death and two were dropouts.
First, univariate analyses for each NMS as well as age, education, sex, and duration of Parkinson’s disease were performed (Table 2). Next, we performed a multivariate binary regression analysis for each NMS that had at least a significance of p<0.1 on univariate analysis as well as all demographic features (adjusting for age, sex, education, and PD duration). A separate analysis was also conducted for levodopa equivalent dose,Reference Hobson, Lang, Martin, Razmy, Rivest and Fleming 9 but given the confounding with duration of PD, it was excluded from the final analysis. A p value of 0.05 was considered statistically significant in this exploratory study.
We studied 48 patients with a mean age of 71.5 and median PD duration of 8.9 years (Table 1). The average MMSE was 28.1 at baseline and decreased to 26.5 at 36 months; the baseline FAB score was 14.5, which decreased to 13.7 at 36 months. Over the course of our study, 20 of 48 (41.7%) patients were deemed to have significant cognitive decline (Table 2). In patients with cognitive decline, the average MMSE score was 27.1 at baseline and 24.0 at 36 months; baseline FAB was 13.7, which decreased to 11 at 36 months. Some 13 of 48 (27%) patients were found to have OH at baseline, with a mean drop in systolic blood pressure of 30.9 mmHg and in diastolic blood pressure of 16 mmHg. A total of 11 of these 13 (84.6%) went on to have clear cognitive decline, whereas 9 of 35 (26%) patients without baseline OH had cognitive decline. Some 11 of 20 (55%) patients with cognitive decline had OH at baseline. Of the 9 patients with cognitive decline without baseline orthostatic hypotension, the mean drop in systolic blood pressure was 0.22, and there was a mean increase of 3.3 in diastolic blood pressure.
Table 1 Testing results at 0 and 36 months
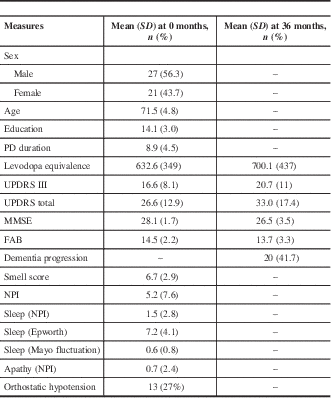
FAB=Frontal Assessment Battery; MMSE=Mini-Mental State Examination; NPI=Neuropsychiatric Inventory; PD=Parkinson’s disease; UPDRS=Unified Parkinson’s Disease Rating Scale
Table 2 Binary univariate and multivariate analysis with cognitive decline as the dependent variable
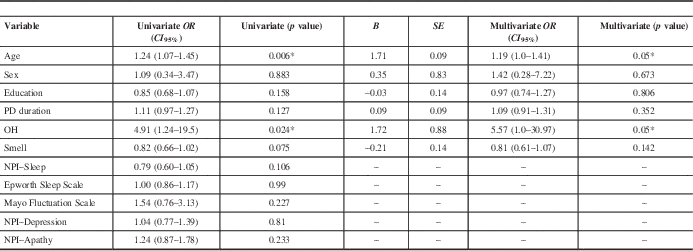
* =statistically significant; NPI=Neuropsychiatric Inventory; OH=orthostatic hypotension; PD=Parkinson’s disease.
In the univariate binary regression analysis with cognitive decline versus no progression as our dependent variable, statistically significant results were found for age (OR (CI 95%)=1.24, 1.07–1.45, p=0.006) and OH (OR (CI 95%)=4.91, 1.24–19.5, p=0.024). Among the NMS, only OH and BSIT smell score (p=0.075) had p values below 0.1, and thus NPI–Sleep score, Epworth Scale score, Mayo Fluctuation Scale score, and NPI–Depression and NPI–Apathy scores (all p>0.1) were excluded from the multivariate analysis (Table 2).
A binary multivariate logistic regression analysis was performed to predict cognitive decline versus no cognitive decline based on the independent variables of OH and BSIT smell scores that were adjusted for age, sex, education, and PD duration. A significant regression equation was found (p=0.007, with a Cox and Snell R 2 and Nagelkerke R 2=0.309 and 0.416, respectively). Compared to the no cognitive decline group, the cognitive decline group was more likely to have baseline OH (OR (CI 95%)=5.57, 1.0–30.97, p=0.05). There was no association with smell scores (OR (CI 95%)=0.81, 0.61–1.07, p=0.142).
In this prospective cohort study, we found that, of the variables selected, other than age, the presence of orthostatic hypotension was the only independent predictor of cognitive change.
This result adds further evidence to the recent literature that showed a link between orthostatic hypotension and cognitive decline.Reference Udow, Robertson and MacIntosh 10 Like the study by Anang et al.,Reference Anang, Gagnon and Bertrand 4 we also did not find neuropsychiatric features or hyposmia to be predictors of dementia. Unlike their study, however, we did not find sleep disturbances—using the modified Epworth Sleepiness Scale, the NPI–Sleep item, and the Mayo Fluctuation Scale—to be significant predictors of cognitive decline. This may be related to the lack of a specified diagnosis of REM sleep behavior disorder in our study. These results are not in keeping with such other studies as Baba et al.,Reference Baba, Kikuchi and Hirayama 2 which appeared to show a link between anosmia and cognitive decline.
Our results support the suggestion that α-synuclein pathology in autonomic centers may precede spread to cortical areas involved in memory and higher cognitive function within a 36-month period.Reference McDonald, Newton and Burn 6 , Reference Udow, Robertson and MacIntosh 10 It remains possible that dysautonomia as measured by orthostatic change might contribute to cognitive decline. Other areas associated with NMS, such as smell and neuropsychiatric features, may still be related, but we were not able to ascertain this within the limited timespan of our study.
The limitations of our study include its short time frame, considering the facts that PD is a chronic neurodegenerative condition, that the presence of pathology is known to occur much earlier in some areas than others, and that changes may progress over time. Dopaminergic medications used might confound our findings, but our analysis, adjusting for duration of disease, was analogous to that of Anang et al.Reference Anang, Nomura, Romenets, Nakashima, Gagnon and Postuma 5 Our study is also limited by its relatively small sample size, which may not provide high enough power to identify some risks that have a weaker association. In addition, our study did not include more direct measures of REM sleep behavior disorder and color discrimination to confirm the findings of recently published data. Nevertheless, our study was prospective, with standardized measures of specific nonmotor symptoms and signs as well as detailed assessment of cognitive decline.
Our study provides further evidence that the presence of orthostatic hypotension is a significant predictor of progression to dementia in PD.




