Introduction
Recent trials have established the benefit of perfusion imaging-based patient selection for endovascular treatment (EVT) in patients with anterior circulation large vessel occlusion (LVO) stroke presenting 6–24 hours after last time seen normal (LTSN). Eligibility was based on varying definitions, relying on imaging criteria (core-penumbral mismatch)Reference Nogueira, Jadhav and Haussen1 or a combination of clinical and imaging findings (core-NIHSS (NIH Stroke Scale Score) mismatch).Reference Albers, Marks and Kemp2 Accordingly, in late-presenting patients (>6 hours from onset or LTSN), imaging, rather than time alone, has become the basis of selection criteria for EVT. While perfusion-imaging methods allow estimations of core and penumbral volumes, they are not available in all stroke centers.
In contrast, standard neuroimaging (consisting of a non-contrast enhanced CT-scan (NCCT) and CT-angiography (CTA)) is widely accessible as part of existing acute ischemic stroke evaluation protocols. Multiple trials have demonstrated the benefit of EVT for patients presenting in the early window (<6 hours from onset or LTSN) based on NCCT and CTA results. The capacity of NCCT and CTA alone to select patients for EVT in the late window has, however, not yet been determined.
The primary aim of the study was to determine the ability of NCCT and CTA to select patients for EVT in the late window. The primary hypothesis was that NCCT and CTA-based selection would lead to similar efficacy and safety outcomes for patients undergoing EVT in the early and late windows.
Methods
Study Design
We conducted a retrospective analysis of all patients undergoing EVT for acute anterior circulation ischemic stroke at a single comprehensive stroke center (CSC) (Centre Hospitalier de l’Université de Montréal (CHUM), Canada) from January 2016 to April 2017. The CHUM performs > 150 thrombectomies annually, mostly following transfers from primary stroke centers (PSCs).
Patients planned for EVT were identified via a prospectively collected electronic patient record system used for acute stroke evaluations. All patients with an anterior circulation stroke undergoing EVT were included. Patients were then classified into early and late window groups (<6 and ≥6 hours from LTSN to CSC). Those in the late window were additionally classified as strokes of known or of unknown onset (i.e., wake-up strokes or non-wake-up unwitnessed onset).
Baseline demographic criteria (age, sex, vascular risk factors, and premorbid modified Rankin scores (mRSs)) and initial NIHSS scores were collected. Standard imaging protocol included an initial NCCT followed by CTA to confirm arterial occlusion. A triple-phase CTA was obtained for all patients presenting directly at our institution, whereas a single-phase CTA was performed in patients evaluated at PSC and subsequently transferred to CSC for EVT. Perfusion imaging (CT-perfusion/magnetic resonance (MR)-perfusion) was not performed for EVT selection during the study period. Eligibility for EVT was at the treating physician’s discretion. Typically, EVT selection at our institution is based on the presence of an intracranial LVO, a significant neurological deficit (NIHSS ≥ 6), and favorable parenchymal imaging (Alberta Stroke Program Early CT Score (ASPECTS) ≥ 6). ASPECT scores were reported as initially documented. Imaging upon CSC arrival was not routinely repeated for patients transferred from PSC. Arterial occlusion as reported on CTA was noted. Standard stroke treatment metrics were calculated. Collaterals were retrospectively evaluated by two raters (CO and FF) and graded from 0 to 4 as defined by Souza et al.Reference Souza, Yoo and Chaudhry3
Patients planned for EVT (based on initial vascular imaging results) but with subsequent documented recanalization on direct angiography were included in the analysis. Recanalization of the artery occluded on initial CTA was reported using the modified Treatment in Cerebral Ischemia (mTICI) score. All CT-scans at 24 hours after EVT were reviewed and 24 hours asymptomatic or symptomatic intracranial hemorrhages (sICHs) (based on European Cooperative Acute Stroke Study III definition) were recorded.Reference Hacke, Kaste and Bluhmki4 mRSs were determined at 3-month clinical follow-up or by telephone interview by certified personnel as part of our existing standard of care for all EVT cases.
Statistics
Continuous variables are reported as means and standard deviations (SDs) or median and interquartile ranges, as appropriate. Dichotomous variables are reported as proportions and were compared by Pearson’s chi-squared test. Clinical outcomes were dichotomized as favorable (mRS 0–2) or unfavorable (mRS 3–6) and compared using Pearson’s chi-squared test. The significance level for two-sided testing of hypotheses was 0.05. SPSS 25 was used for statistical analyses.
Ethics
Retrospective chart review was approved by our medical institutional review board for all EVT cases.
Results
Baseline Population Characteristics
Over the study period, a total of 248 patients underwent EVT, 204 (82%) <6 hours, and 44 (18%) ≥6 hours from LTSN. Baseline characteristics including age, sex, and vascular risk factors did not differ between groups (Table 1).
Table 1: Baseline characteristics of patients undergoing EVT
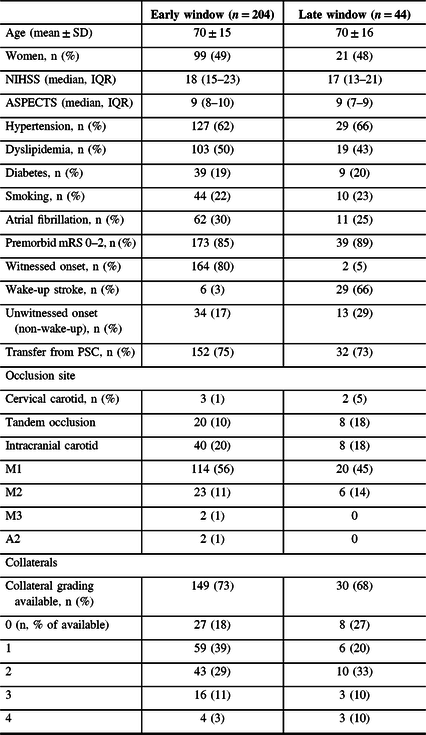
Most patients treated by EVT were transfers from PSC in the early (152 (75%)) and late (32 (73%)) windows. Median (IQR) LTSN to CSC time was 188 (134–242) minutes in the early and 586 (430–799) minutes in the late window (difference: 398 minutes). Median (IQR) first time seen symptomatic to CSC time was 170 (122–210) minutes in the early and 197 (152–282) minutes in the late window (difference: 27 minutes) (Table 2). The late window group mainly consisted of patients with strokes of unknown onset (42 (95%)) rather than late-presenting strokes (2 (5%)). Amongst late window strokes of unknown onset, 29/42 (69%) were wake-up strokes.
Table 2: Recanalization therapy in patients undergoing EVT
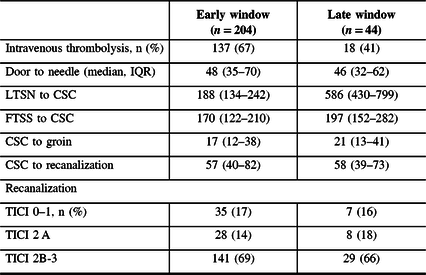
FTSS = first time seen symptomatic.
Initial median (IQR) NIHSS scores were similar (early: 18 (15–23) vs. late: 17 (13–21)), as were median ASPECT scores (early: 9 (8–10) vs. late: 9 (7–9)). Collateral status was available in 149 (73%) patients in the early and 30 (68%) in the late window. The proportion of patients with poor collateral status (grade 0–1) was slightly higher in the early (86/149 (58%)) than in the late (14/30 (47%)) window.
Recanalization Therapy and Functional Outcomes
Thrombolysis was more frequently administered to early-presenting patients (137 (67%) vs. 18 (41%)). Standard stroke treatment metrics, including door-to-needle, CSC-to-groin, and CSC-to-recanalization, did not differ (Table 2). Proportions of favorable recanalization (mTICI 2b-3) were similar in the early (141 (69%)) and late (29 (66%)) windows.
Safety Outcomes
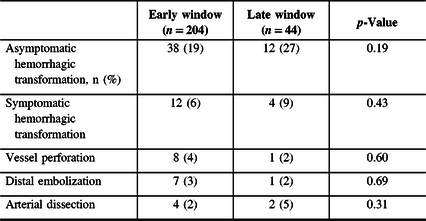
The proportions of sHT at 24 hours did not differ in the early (12 (6%)) and late (4 (9%)) windows (p = 0.43). When combining 24-hour symptomatic hemorrhagic transformation (sHT) and asymptomatic hemorrhagic transformation, more events occurred in the late window (early vs. late: 50 (25%) vs. 16 (36%)), but this did not reach statistical significance (p = 0.11) (Table 3).
Table 3: Adverse event in patients undergoing EVT
Three-Month Outcomes
Among patients with available 3-month mRS scores (early: 192/204 (94%) and late: 41/44 (93%)), the proportions achieving functional independence (mRS 0–2) were similar (early: 80 (42%) vs. late: 16 (39%), p = 0.72) (Figure 1).

Figure 1: Three-month modified Rankin scores (mRSs) after endovascular therapy (EVT) in the early and late windows.
Discussion
In this study, we found that EVT selection based on standard neuroimaging resulted in similar proportions of functional independence in both early and late windows, with no significant increase in hemorrhagic transformation. These results are concordant with previous studies retrospectively evaluating NCCT- and CTA-based late window stroke patient selection for EVT.Reference Santos, Carvalho and Cunha5,Reference Motyer, Thornton and Power6 These findings specifically apply to patients with strokes of unknown onset, as they formed almost all the late window group.
American Heart Association guidelines recommend strict application of the DAWN and DEFUSE-3 perfusion-based criteria for patient selection if LTSN is ≥ 6 hours, as no other eligibility criteria have been prospectively validated.Reference Powers, Rabinstein and Ackerson7 Canadian guidelines make the same recommendation for EVT admissibility in the late window.Reference Boulanger, Lindsay and Gubitz8 However, no recommendations are made for centers without access to perfusion neuroimaging.
Late window LVO stroke selection strategies should seek to identify patients for whom the estimated benefit of recanalizing penumbra offsets the risk associated with hemorrhagic transformation and other complications of EVT. Perfusion-based trial’s inclusion criteria allowed for moderate core volumes (up to 21–51 mL in DAWN and 70 mL in DEFUSE-3). However, EVT-treated patients had significantly smaller median estimated core volumes (7.6 mL in DAWN and 9.4 mL in DEFUSE-3, with a median estimated penumbra of 114.7 mL). Haussen et al. established a fair correlation between ASPECTS and CT-perfusion results.Reference Haussen, Dehkharghani and Rangaraju9 NCCTs with an ASPECTS ≥ 6 had a CT-perfusion median core volume ≤ 20 mL, and most were ≤50 mL. Another study found no difference between ASPECTS and CT-perfusion’s accuracy for hyperacute MRI diffusion lesion volume prediction.Reference Demeestere, Garcia-Esperon and Garcia-Bermejo10 Further data suggest higher inter-rater agreement of ASPECTS with increasing time from stroke onset, making the score more reproducible in the late window.Reference Naylor, Churilov and Chen11 On the other hand, while NCCT might be able to estimate core volume, it cannot measure penumbra. Clinical-radiological mismatch could serve as a surrogate for penumbra, particularly with very proximal occlusions (terminal internal carotid artery or M1) and high ASPECT scores (≥8), suggesting sizeable brain regions at risk and small core volumes.
In this study, late window LVO stroke patients selected with NCCT achieved similar 3-month functional independence outcomes as patients in the MR CLEAN trial.Reference Berkhemer, Fransen and Beumer12 These results suggest that in centers where perfusion neuroimaging is not available, NCCT and CTA might accurately select late window EVT candidates, particularly for strokes of unknown onset. This also applies to PSC where automated perfusion software might not be affordable nor justifiable due to low LVO stroke volume, but who nevertheless see patients amenable to EVT. In these cases, repeating perfusion imaging at CSC after transfer might lead to further delays and increase radiation exposure. The ESCAPE trial included patients up to 12 hours from symptom onset using NCCT and CTA results, excluding patients with poor collateral status.Reference Goyal, Demchuk and Menon13 A subgroup analysis subsequently showed no heterogeneity in EVT effect in the early and late windows.Reference Goyal, Demchuk and Menon13,Reference Evans, Graham and Pordeli14 However, the 39% proportion of mRS 0–2 outcomes observed in this study is lower than the 49% in DAWN and 44% in DEFUSE-3. Baseline NCCT ASPECT score modified the effect of EVT in the DAWN trial,Reference Bhuva, Yoo and Jadhav15 but it has not been prospectively demonstrated that late window patients with a favorable ASPECT score alone benefit from EVT.
Two-thirds of the late window patients in this study were wake-up strokes. Epidemiological data suggest that, in Canada, 14% of strokes occur at wake-up.Reference Nadeau, Fang and Kapral16 Silva et al. report similar infarct volumes and CT-perfusion cerebral blood flow-cerebral blood volume mismatch rates in strokes of known onset and wake-up strokes.Reference Silva, Lima and Camargo17 A significant proportion of wake-up strokes likely occur near awakening. In a secondary analysis of the DAWN trial, benefit of EVT was independent of the mode of presentation.Reference Jadhav, Aghaebrahim and Jankowitz18 Trials should separate wake-up strokes from other unknown onset and late presenting strokes, and the impact of recanalization therapy should be differentially evaluated in these subgroups.
Prior studies support the notion that NCCT and CTA can safely select wake-up stroke patients for reperfusion therapy. Hill et al. thrombolyzed 20 wake-up stroke patients (median ASPECTS = 9) with no incident sICH.Reference Hill, Kenney and Dzialowski19 Bal et al. documented no sICH amongst a cohort of 29 moderately severe wake-up stroke patients (median ASPECTS = 7) who received recanalization treatment.Reference Bal, Bhatia and Shobha20 Efficacy of intravenous thrombolysis for late-window patients has been demonstrated in the WAKE-UP and EXTEND trials, where the benefit of recanalization offset the 1.6–5.3% increase in sICH in the alteplase versus placebo groups.Reference Thomalla, Simonsen and Boutitie21,Reference Ma, Campbell and Parsons22 These results and our local experience confirm the notion that time is a single variable that must be integrated with imaging results and patient characteristics for recanalization treatment candidate selection.
Limitations of this study include its small size, single-center retrospective design, and use of patients LTSN < 6 hours as the control group, as patients with favorable NCCT profiles in the late window might represent a select subset of cases with better penumbral tolerance to ischemia (i.e., the late window paradox).Reference Albers23 Most patients were strokes of unknown onset, and results are not generalizable to late-presenting strokes of known onset. Collateral grading/triple phase CTA was not available in all cases. Proportions of poor collateral status and occlusion sites varied in the early and late windows, which may have differentially influenced outcomes. This study might have been underpowered to detect a statistically significant difference in adverse events. NCCT and CTA results as well as outcomes were not available for late window patients who were evaluated but not selected for EVT, limiting our finding’s generalizability, as other undocumented factors might have influenced the decision to perform EVT. Without documentation of these cases, it was impossible to determine the proportion of late-presenting patients considered eligible for EVT based on NCCT and CTA results.
Conclusion
Late-window LVO anterior circulation stroke patients selected for EVT using NCCT and CTA achieved similar 3-month functional outcomes to early presenting patients, with no increase in sHT. In the late window, NCCT- and CTA-based patient selection for EVT appears safe and could be an alternative to perfusion neuroimaging, especially for strokes of unknown onset with high ASPECT scores. Prospective studies comparing both approaches are warranted.
Disclosures
M-CB, AN, FF, FK, ND, GJ, CS, and CO report no conflicts. YD reports personal fees from Servier, outside the submitted work. LCG reports grants and personal fees from Servier, personal fees from Bayer, personal fees from Pfizer, and personal fees from Bristol-Myers Squibb, outside the submitted work. AYP reports research support from Servier and Bayer, outside the submitted work.
Statement of authorship
M-CB and AN designed and conceptualized the study, collected data, performed analysis and interpretation of the data, and drafted the manuscript. FF reviewed imaging and revised the manuscript. FK, ND, YD, LCG, GJ, AYP, and CS provided data and revised the manuscript. CO designed and conceptualized the study, collected data, reviewed imaging, and revised the manuscript.






