Introduction
Mercury (Hg) is a nonessential element for the human body. Hg is emitted into the environment and released into landfill and water systems from anthropogenic activities or natural sources. Expanded industrial use of Hg has exposed a large population to its neurotoxic effects. Hg exists as elemental (vapor), inorganic (mercuric salts, when elemental mercury combines with sulfur, chlorine), and organic salts (when elemental mercury combines with carbon, e.g. methylmercury (meHg)). Reference Rafati-Rahimzadeh, Rafati-Rahimzadeh, Kazemi and Moghadamnia1 Elemental mercury affects humans via occupational (Hg vapor) or environmental exposure (air pollution), whereas MeHg comes mainly from the aquatic marine food chain. As our society has advanced, modes of human exposure to Hg have changed, but it is still very much relevant and necessary not to forget this as a risk factor for movement disorders like cerebellar ataxia, tremor, and myoclonus. Failure to recognize this toxic etiology often leads to unnecessary investigations and misdiagnosis. There is also evidence suggestive of the possible link of Hg with parkinsonism and autoimmune movement disorders. The studies on Hg and movement disorders have investigated heterogeneous patient populations with a diverse source of exposure. In this review, we have consolidated the literature on human exposure to Hg and movement disorders, along with their pathophysiological basis and the preventive strategies.
Literature Search
The literature search was conducted using four databases: PubMed, ScienceDirect, Cochrane Library, and BioMed Central (January 1930 to December 2020) using keywords “mercury and neurology”, “mercury and movement disorders”, “mercury and tremor”, “mercury and ataxia”, and “mercury and parkinsonism”. Duplicates were removed. Articles highlighting mainly the movement disorder perspective from human exposure to Hg were included. A consolidated overview has been extracted from them and presented in this review.
In the following sections, we have first discussed biochemical properties of Hg and pathophysiology of neurotoxicity, followed by major sources of human exposure and movement disorders related to Hg exposure.
Biochemistry and Pathophysiology
Elemental mercury (Hg0) vaporizes at room temperature and enters into the body via an inhalational route. It can cross the blood–brain barrier (BBB) to cause neurotoxicity. In the bloodstream, Hg0 is oxidized to mercuric ion (Hg2+) by the catalase enzyme. Hg2+ by itself cannot cross the BBB, though it can interact with intracellular enzymes, transporters, ion channels, and glutathione and paralyze the detoxification machinery. Urine and feces are the main excretory routes for elemental mercury. Reference Martinez-Finley and Aschner2,Reference Clarkson, Vyas and Ballatori3 Organic MeHg, on the other hand, intoxicates the marine ecosystem and affects humans mainly via the aquatic food chain. MeHg is almost 95% absorbed from the intestine. Subsequently, it forms complexes with thiol (-SH) and selenol (-SeH) groups and circulates to various organs including the central nervous system. MeHg-L-cysteine complex is transported across the BBB by the L-type amino acid transporters and is the main culprit for neurotoxic effects. Reference Clarkson, Vyas and Ballatori3 In the brain, glutathione complexes with MeHg for detoxification. MeHg complex gets excreted through the bile, kidney, and finally as mercuric Hg via feces [Figure 1]. Human exposure to elemental and organic mercury is highlighted in Figure 2.

Figure 1: Uptake and transport of mercury in the body.

Figure 2: Human exposure to elemental and organic mercury.
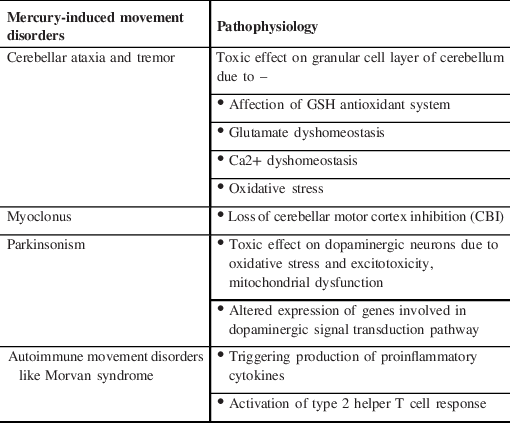
MeHg-induced neurotoxicity is mainly related to four interrelated cellular events: (i) affection of GSH antioxidant system; (ii) glutamate dyshomeostasis; (iii) Ca2+ dyshomeostasis; and (iv) increased reactive oxygen species (ROS) generation and oxidative stress. Reference Farina, Rocha and Aschner4 MeHg is highly toxic to the granule cells in the cerebellum while the Purkinje cells are relatively refractory. Though this specificity for granule cell pathology is unclear, differential expression of GABAA receptor subunits (α6 GABAA receptor subunits on granule cells and α1 on the Purkinje cells) and NMDA subunits have been postulated (higher NMDAR2 expression in granule cells and NMDAR1 in Purkinje cells) as the likely causes. Reference Yuan and Atchison5,Reference Kaur, Aschner and Syversen6
Hg-induced oxidative stress, disruption of neurotransmitter metabolism, excitotoxicity, neuroinflammation, selenium depletion, interaction with microtubules, altered membrane transport, and genetic susceptibility are hypothesized as possible etiological factors for neurodegeneration due to Hg exposure. Reference Bjørklund, Dadar, Mutter and Aaseth7–Reference Andrew, Chen and Caller10 In mouse models, MeHg has been shown to decrease the number of neurites and alter the cytoskeletal structure in dopaminergic neurons. Reference Götz, Koutsilieri, Riederer, Ceccatelli and Daré11 Using a dopaminergic cell line, Shao et al. have demonstrated that MeHg can affect dopaminergic signaling in a way similar to 1-methyl-4-phenylpyridinium (MPP+). Reference Shao, Figeys, Ning, Mailloux and Chan12 MPP+ and MeHg both can alter the expression of genes involved in dopaminergic signal transduction pathway like dopa decarboxylase (DDC), PARK2, PARK7, SLC6A3, synuclein alpha (SNCA), and tyrosine hydroxylase (TH).
Hg can also trigger the production of proinflammatory factors like interferon-gamma (IFN-γ), tumor necrosis factor (TNF)-α, interleukin 1 β (IL-1 β), and autoantibodies. Reference Pollard, Cauvi, Toomey, Hultman and Kono13 Loss of tolerance to self-antigens, change in autoantigen fibrillarin, activation of type 2 T helper cell (Th2) response, production of proinflammatory cytokines, stimulation of Cathepsin B activity, and induction of VEGF and IL-6 have all been attributed to the possible link between Hg and autoimmunity. Reference Bjørklund, Peana, Dadar, Chirumbolo, Aaseth and Martins14 Genetic polymorphisms in Glutathione S-transferase family (GSTs), metallothioneins (MTs), selenoproteins, ATP-binding cassette transporter superfamily (ABCs), organic anion transporters (OATs), L-amino acid transporters (LATs), and APOE ⊠4 allele are likely responsible for differential body handling of Hg in susceptible individuals after environmental exposure. Reference Andreoli and Sprovieri15 Pathophysiology of Hg-induced movement disorders has been summarized in Table 1.
Table 1: Pathophysiology of mercury-induced movement disorders
Human Exposure to Mercury
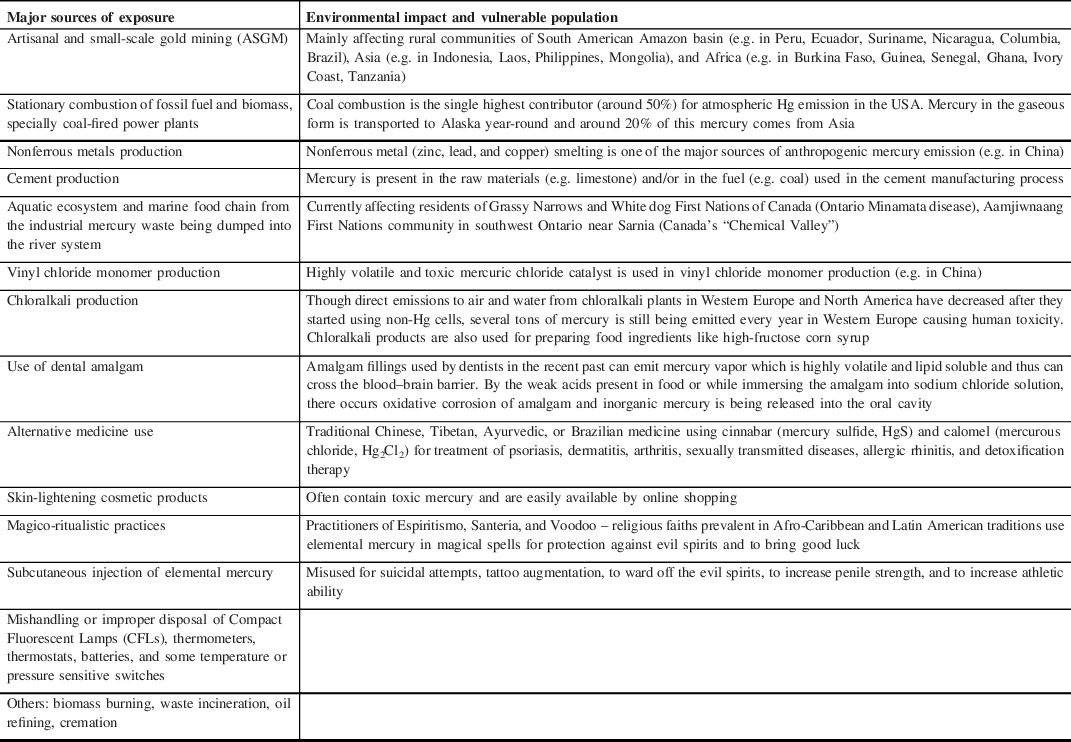
The use of Hg can be found in the history of medicine and alchemy of the ancient Egyptian, Greek, Roman, Chinese, and Hindu civilizations. Reference Norn, Permin, Kruse and Kruse16 Hg was used by the ancient Egyptians and archeologists have found Hg in an Egyptian tomb dating to 1500 BC. The alchemists wondered about the mystical properties of Hg and used it to transmute base metals into gold. For alchemy, the Hindus used the word “Rasasiddhi” that means “knowledge of mercury”, with a belief that Hg was at the core of all metals and correct combination of Hg and other ingredients can yield gold. Gradually, the distinction between alchemy and medicine became blurred and cinnabar (mercuric sulfide) started being misused as an elixir of life to confer longevity or immortality. Reports of the therapeutic use of Hg and its compounds in syphilis are available from the 15th century till as late as the mid 20th century. Reference Graeme and Pollack17 With the advent of industrialization in 19th century, the toxic effects of Hg started affecting humanity in different parts of the world [Table 2]. An age-old source of Hg intoxication is the amalgamation technique, mainly used for gold extraction and the tradition is still being followed in many countries. The ore on being mixed with water, salt, and liquid Hg bind to the metal as an Hg–gold amalgam complex that releases Hg vapors if heated, leaving behind the gold particles. These vapors are an important source of inhalational Hg toxicity from ancient times and the metallic form is released into the rivers and carried downstream, becoming another major source of toxicity. Reference Brooks, Öztürk and Cansu18,Reference Brooks, Schwörbel and Castillo19 Aquatic ecosystem and marine food chain are getting affected by the toxic waste of Hg even today. The Global Mercury Assessment 2018 (GMA 2018) conducted by the United Nations has shown that artisanal and small-scale gold mining (ASGM) (37.7%), stationary combustion of fossil fuel and biomass, especially coal (21%), nonferrous metals production (15%), and cement production (11%) are currently the four main sources of Hg emission. Major sources of Hg exposure in the modern world are shown in Table 3.
Table 2: Historical vignettes of mercury exposure
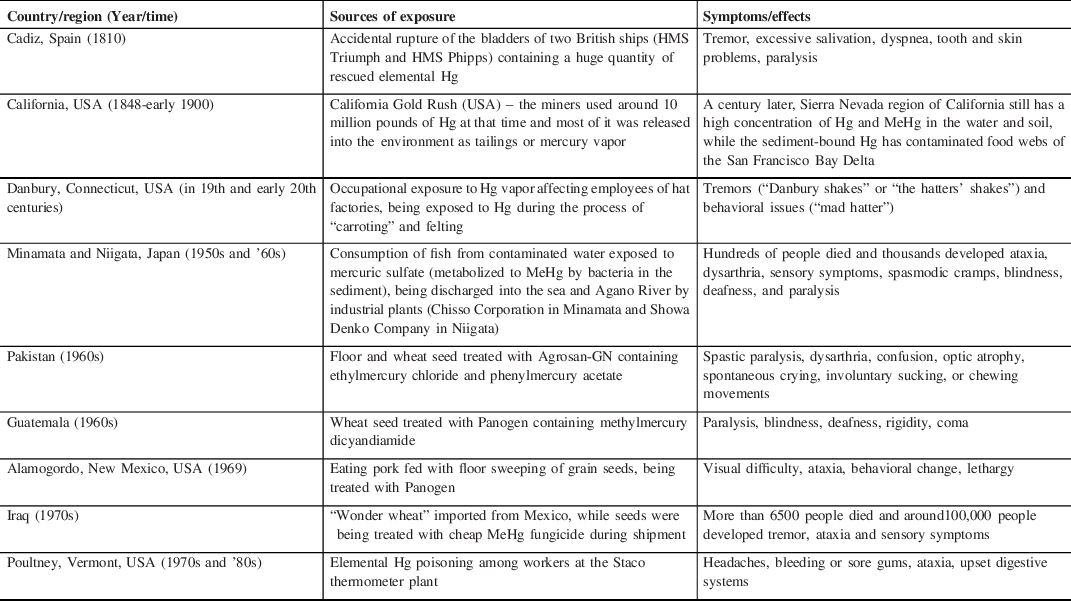
Table 3: Present global scenario of mercury exposure
Human exposure to Hg can have toxic effects on cardiovascular (cardiomyopathy), hematological (hemolytic and aplastic anemia), renal (acute tubular necrosis, nephrotic syndrome, glomerulonephritis, chronic kidney disease), pulmonary (bronchitis, pulmonary fibrosis), immunological (affecting polymorphonuclear leukocytes, flaring up autoimmunity), endocrinal (hypoadrenalism, hypopituitarism, diabetes mellitus, hypothyroidism), reproductive (reduced fertility, menstrual disorders), embryonic (neural tube defects, craniofacial malformations, growth retardation), and neurological (movement disorders, psychiatric issues, autism, peripheral sensory neuropathy, optic neuropathy, hearing impairment) systems. Reference Rice, Walker, Wu, Gillette and Blough20 In this review, we have highlighted the neurotoxicity of Hg from a movement disorder perspective.
Movement Disorders Associated with Mercury Exposure
-
1. Named syndromic disorders:
-
(a) Hunter Russell syndrome and Minamata disease: In the early 1950s, an epidemiological study in Minamata, Japan first noticed that several cats fed on a fish diet were developing movement disorders, convulsions, and death (“dancing cat disease”). Reference Kitamura, Miyata and Tomita21 In 1977, a similar incident was noted in Northwestern Ontario, Canada where cats were noted to develop ataxic gait, movement disorders, seizures, and uncontrolled howling. Reference Takeuchi, D’Itri, Fischer, Annett and Okabe22 Cerebellar ataxia, dysarthria, and visual field constriction constitute the classic triad of Hunter-Russell syndrome of organic Hg intoxication. Reference Hunter and Russell23 Postural and action tremor were prominent. Electrophysiological analysis of the postural tremor in Minamata disease and have noted the mean frequency of 7.1 Hz and mean amplitude of 1.8 mV. Reference Yamanaga24 Brain autopsy of patients with “Minamata disease” showed degeneration and damage to the calcarine region of the occipital lobe, central and temporal cortices, atrophy of the granular cell layer of cerebellum, and dorsal roots. Reference Eto, Marumoto and Takeya25
-
(b) Danbury shakes: In the 19th and early 20th centuries, workers of hat factories at Danbury, Connecticut, USA, exposed to Hg vapor during the process of “carroting” and felting, were noticed to have fine tremor in hands interrupted by coarse myoclonic jerks (“Danbury shakes”) Reference Tepper26 along with the behavioral issues ranging from pathological shyness to profound irritability, insomnia, hyperactivity and psychotic behaviors (“mad hatters”). Jerky postural tremor is one of the commonest presentations of elemental Hg poisoning and has been described in the literature as “mercurial tremors”, “metallic tremor” or “tremblant metallique”, the term used to describe the tremor noted in water gilders in Paris.
-
-
2. Other movement disorders
-
(a) Cerebellar ataxia and tremor: Gait and limb ataxia, dysmetria, and dysdiadochokinesia along with dysarthria and visual field constriction were reported in lamp socket manufacturers chronically exposed to elementalHg. Reference Yang, Huang, Shih and Yang27 Rhee et al. have reported subacute onset slowly progressive cerebellar ataxia from exposure to Hg vapor. Reference Rhee, Lee, Yoon, Lee, Chang and Chang28 Subacute cerebellar ataxia has also been reported from the Indian subcontinent from ayurvedic medicine containing a high amount ofHg. Reference Renjen and Chaudhari29 Malkani et al. have reported the development of limb and gait ataxia, dysdiadochokinesia, nystagmus, dysarthria along with multiple cranial nerve palsies from subcutaneous injection of elemental Hg (“quick silver pellets”) with the cultural belief of protection against sickness. Reference Malkani, Weinstein, Kumar, Victor and Bernstein30
-
A study in Brazilian gold traders exposed to Hg vapor have measured tremor frequency mainly in high-frequency windows (6.6–10.0 Hz). Reference Biernat, Ellias and Wermuth31 Motor deficits in the form of tremor, incoordination and poor fine motor control, and subtle cognitive changes have been noted among dentists exposed toHg. Reference Echeverria, Heyer, Martin, Naleway, Woods and Bittner32 A study by Rustam et al. on MeHg poisoning in Iraq, noted that around 13% of patients had intention tremor, while flapping and static tremor were less frequent. Reference Rustam and Hamdi33 Sequential neurological manifestations have been reported in Hg vapor (elemental mercury) poisoning. While initial symptoms can be upper limb weakness, intention tremor, loss of fine motor coordination, and numbness, continued exposure leads to coarser tremor and spasm interfering with feeding or writing. Tremor and spasm can later involve the lower extremity, trunk, and head. Reference Doherty34 Schaumburg et al. have reported the development of mild fine rapid rest tremor of the fingers, markedly exaggerated on movement, in a patient with a history of self-administration of elemental mercury subcutaneously. Reference Schaumburg, Gellido, Smith, Nelson and Hoffman35 Bradberry et al. have reported a case of a 36-year-old jewelry producer exposed to Hg vapor presenting with a coarse tremor of the hands and protruded tongue, slurred speech, cerebellar ataxia, and constricted visual fields. Reference Bradberry, Sheehan, Barraclough and Vale36
-
(b) Myoclonus: Roullet et al. have reported severe intention and action myoclonus in a 58-year-old laboratory glassware manufacturer with occupational exposure to Hg vapor. Reference Roullet, Nizou, Jedynak and Lhermitte37 Ragothaman et al. have described a case of a 23-year Chemistry graduate, who developed progressive generalized myoclonus and ataxia as a result of self-injecting elemental mercury. Reference Ragothaman, Kulkarni and Ashraf38 Myoclonus was present at rest and worsened with activity. Electrophysiological studies revealed large-amplitude (12.1 µV) somatosensory-evoked potential (SSEP) and high frequency (10–30 Hz), short duration (less than 50 ms) EMG bursts, suggesting the cortical origin of the myoclonus. The symptoms improved after surgical clearance of the subcutaneous Hg deposits from the injection site.
-
(c) Chorea: Ko et al. have reported the development of generalized chorea from Hg poisoning in a 50-year-old female, in whom symptoms improved with chelation therapy. Reference Ko, Lee and Kandg39 Chorea, athetosis, and hemiballismus were reported in patients exposed to MeHg in Japan and Iraq. Reference Rustam and Hamdi33,Reference Ekino, Susa, Ninomiya, Imamura and Kitamura40–Reference Snyder42
-
(d) Parkinsonism: Hsu et al. noted that in patients exposed to dental amalgam fillings, the incidence of PD was 1.5 times higher than those nonexposed. Reference Hsu, Chang and Lee43 Some studies have also noted a higher incidence of PD in dentists when regularly exposed to Hg vapor. Reference Schulte, Burnett, Boeniger and Johnson44,Reference Bjorklund, Stejskal, Urbina, Dadar, Chirumbolo and Mutter45 Finkelstein et al. reported the development of hemiparkinsonism in a 47-year-old female dentist who improved with chelation therapy by D-Penicillamine. Reference Finkelstein, Vardi, Kesten and Hod46 Fabrizio et al. have noted a high prevalence of extrapyramidal signs and symptoms (PD, postural tremor) in a group of male dental technicians working in a technical high school in Rome. Reference Fabrizio, Vanacore, Valente, Rubino and Meco47 Miller et al. have reported a case of a 55-year-old worker of chlorine factory, who developed parkinsonism after chronic exposure to metallic Hg vapor. Reference Miller, Ochudło, Opala, Smolicha and Siuda48 Palacios et al. have investigated a possible correlation between airborne metal exposure of female nurses and risk of PD by a large prospective study with a study duration of 18 years (1990–2008) and involving 121,701 participants from 11 different US states. Reference Palacios, Fitzgerald and Roberts49 In the study, Hg was the only metal found to be associated with the highest risk of PD(CI = 0.99–1.79). A study by Dantzig Reference Dantzig50 has also shown the possible relation between PD and chronic low levels of Hg poisoning in susceptible individuals. Reference Dantzig50 The study noted that detectable blood Hg levels were six times more frequent in individuals with PD than in healthy controls and proposed that a dermatological manifestation of chronic Hg exposure, Grover’s disease (transient acantholytic dermatitis), may be a clinical clue to predict who are at risk of developing PD. Lin et al. showed a significant decrease in striatal dopamine transporter (DAT) in workers exposed to Hg vapor. Reference Lin, Liou, Hsiech, Ku and Tsai51 However, unless a definite correlation between chronic Hg exposure and development of PD is shown in large-scale human studies, the possible link between Hg and PD remains a matter of debate.
-
-
3. Mercury and autoimmune movement disorders
Recently, Pérez et al. have summarized 41 cases of Hg-induced Morvan syndrome, the autoimmune disorder related to antibodies against contactin-associated protein-like 2 (Caspr2). Reference Pérez, Shah and Butler52 Features of peripheral nerve hyperexcitability like neuromyotonia, electromyographic evidence of myokymia along with encephalopathy, insomnia, and autonomic dysfunction are the hallmarks of Morvan syndrome. Additional painful paresthesia, severe myalgia, arthralgia, and generalized rash were noted by Pérez et al. in “Morvan syndrome-plus” related to an accidental indoor spill of metallic Hg. In addition to the characteristic anti-Caspr2 antibody, other antibodies like anti-leucine-rich glioma-inactivated protein 1 (LGI1), anti-glutamic acid decarboxylase 65-kilodalton isoform (GAD65), and anti-voltage-gated calcium channel (VGCC) antibodies were detected by Pérez et al., suggesting larger spectrum of autoimmune response to Hg. In the literature, varied source of exposure to Hg and resulting Morvan syndrome-like phenotype with serum anti-Caspr2 positivity has been described. Like accidental spillage of a bottle of Hg beads, therapeutic use of mercuric iodide, use of skin whitener, and taking indigenous or ayurvedic medicine. Reference Lu and Khera53–Reference Perumal, Velayudham, Jeyaraj and Arunan57 Similarly, Malkani et al. noted anti-Purkinje cell cytoplasmic-type Tr antibody positivity in a case of elemental mercury toxicity presenting with cerebellar ataxia. Reference Malkani, Weinstein, Kumar, Victor and Bernstein30 In a developing country like India, ayurvedic drugs containing Hg are still being used abundantly and the physician should never forget to take detailed drug history when a patient presents with such phenotype.
-
4. Other neurological features associated with mercury-induced movement disorders
Some neurological features apart from movement disorders are often associated with elementary and organic Hg exposure and can be vital clues for the clinicians to suspect this toxic etiology. Neuropsychiatric symptoms of chronic elementary Hg exposure can range from irritability, insomnia, excessive dreaming to the full-blown picture of “erythrism” in the late stage with personality changes, excessive shyness mixed with nervousness and irritability, anxiety, depression, delirium, hallucination, and seizures. Reference Stier and Gordon58,Reference Lucchini and Hashim59 Dyspnoea, hypersalivation, skin ulceration, and tooth loss are other clues that can indicate elemental Hg poisoning.
On the other hand, moderate to severe constriction of the bilateral visual fields is one of the characteristic features of MeHg poisoning along with sensory impairment in distal extremities in the form of paresthesia and numbness. Reference Bakir, Damluji and Amin-Zaki60 Perioral sensory disturbance with an “onion peel” somatosensory distribution can be seen. Reference Jackson61,Reference Tokuomi, Uchino, Imamura, Yamanaga, Nakanishi and Ideta62 Hearing difficulty, dizziness, and unsteadiness are commonly associated. Reference Yorifuji, Tsuda, Takao and Harada63,Reference Tsubaki and Irukayama64
Management of Suspected Mercury Exposure/Toxicity
Laboratory Investigations
Detailed history including occupational exposure and thorough clinical examination still hold the key in diagnosing Hg-related movement disorders. Any exposure to indigenous or ayurvedic medicine and any practice of magico-ritualistic use of Hg should not be overlooked either. Laboratory investigations to detect the presence of Hg intoxication include blood and urine Hg levels, assays on scalp hair and toenail samples. Reference Ye, Kim and Jeon65 A literature review by Fields et al., comprising over 3000 workers chronically exposed to elemental Hg, has mentioned that physical examination (tremor, incoordination, brisk reflexes) is more important for assessing workers with urinary Hg level > 200 µg/L, while for those with low urine Hg levels, neurobehavioral testing is more useful. Reference Fields, Borak and Louis66 However, the slow excretion of Hg from the body and non-accuracy to predict the timing of neuronal injury make these assays not very helpful in Hg-induced neurotoxicity. Lack of adequate dose–response studies makes it difficult to set a threshold level for Hg in blood or urine for immunologically sensitive individuals.
Chelation Therapy
D-Penicillamine has been used as an oral chelating agent in inorganic Hg toxicity (adult dose: 250 mg qid, pediatric dose: 20–30 mg/kg/day for 1–2 weeks), but has no role in MeHg exposure. Reference Gledhill and Hopkins67,Reference Caravati, Erdman and Christianson68 Hypersensitivity and nephrotoxicity are the commonest side effects of penicillamine. Similarly, dimercaprol or British anti-Lewisite (BAL) has also been used successfully mainly in inorganic and elemental mercury poisoning (deep intramuscular injection of 5 mg/kg for every 4 h for 1–2 days, then 2.5 mg/kg one–two times/day for 10 days). Reference Renjen and Chaudhari29,Reference Kamensky, Horton, Kingsley and Bridges69 Nausea, vomiting, hypertension, tachycardia, headache, diaphoresis, and convulsions are the common side effects of dimercaprol.
The water-soluble analogues of BAL, 2,3-dimercaptopropane sulfonic acid (DMPS), and Meso 2,3-dimercaptosuccinic acid (DMSA) are better alternatives for detoxification in elemental or organic Hg exposure. Reference Kosnett70 They can be administered orally or as an IV injection. In the Mt. Diwata study (Philippines), gold miners exposed to elemental mercury were compared to people living downstream who were exposed to MeHg from eating fish and to controls without known exposure toHg. Reference Böse-O’Reilly, Drasch and Beinhoff71 One-hundred and six probands completed the 2-week trial of oral DMPS 400 mg daily. The study noted a marked improvement in hypomimia, pencil tapping, Romberg test, tremor, and ataxia. Importantly, the therapeutic efficacy was almost similar between the participants exposed to the elemental mercury group (miners) and in the MeHg group (downstream fish eaters). In another study, among patients with occupational exposure to Hg vapor, 2 weeks of oral DMPS 100 mg thrice daily followed by DMPS 100 mg four times a day for an additional 6 weeks have shown beneficial effects. Reference Campbell, Clarkson and Omar72 Dose of DMSA is 10 mg/kg (350 mg/m2 in children) tid for the initial 5 days, followed by 10 mg/kg (350 mg/m2 in children) bid for the next 14 days. Reference Rafati-Rahimzadeh, Rafati-Rahimzadeh, Kazemi and Moghadamnia1 Newer DMSA analogues like Mono isoamyl ester of DMSA (MiADMSA), Monomethyl DMSA (MmDMSA), and Monocyclohexyl DMSA (MchDMSA), either alone or in combination with DMSA, are also being studied for better chelation therapy. Reference Bhadauria and Flora73
Preventive Measures for Mercury Contamination
Though the overall Hg emissions into the environment have decreased over the past decades, improper disposal of industrial waste, artisanal gold mining, and indiscriminate use and more importantly disposal of Hg-containing products like dental amalgam, CFLs, batteries, etc., are still polluting our ecosystem and affecting us via different routes. Minamata Convention on Mercury, a global treaty, has been formed to address these issues. Different preventive measures can be taken to minimize the Hg contamination like: (i) maintaining proper protocol for transport and handling of Hg products; (ii) installation of ISO-11143-certified amalgam separators; (iii) using Hg-free dental materials; (iv) using carbon nanotubes (CNTs) to remove Hg from water; (v) operationalizing proper collection and recycling programs for Hg-containing lamps; (vi) stopping the use of Hg cells in chloralkali plants and upgrading them to newer “membrane technology”; (vii) substitution by non-Hg alternatives in residential or industrial sectors; (viii) restricting the use of Hg in gold mining and using other techniques like “concentration methods”, “direct smelting”, or “cyanide leaching” to extract gold; (ix) controlling Hg emissions through “end-of-pipe techniques” like exhaust gas filtering and using controlled landfill for waste disposal; (x) proper vigilance on the misuse of Hg in indigenous medicine available in the market or online; and (xi) prohibition of the misuse of Hg in magico-ritualistic practices. Reference Rafati-Rahimzadeh, Rafati-Rahimzadeh, Kazemi and Moghadamnia1,74–Reference Valderrama77 The measures need to be strictly implemented and monitored. More active involvement of the groups such as the United Nations Environment Programme or the Artisanal Gold Council is needed to teach the miners to incorporate relatively inexpensive technologies into their work so that they can sustain their livelihood and perhaps enjoy a better life with less exposure toHg. Reference Konkel78 Finally, trading in hazardous wastes like ship breaking industry, though provides raw materials as an incentive to the weaker economy of developing countries, also adds to toxic wastes that have serious implications on environmental and human health. Though the 1989 Basel convention seeks to halt such transport of hazardous wastes, illegal trade and exports between less regulated countries persist. Reference Sonak, Sonak and Giriyan79 There should be means to recycle or reuse this waste to prevent toxic exposure to the native population and workers.
Conclusion
Toxic effects of Hg have affected humanity through the pages of history. Modernization of society has changed modes of human exposure, but Hg is still a potential etiology in patients presenting with cerebellar ataxia, postural and action tremor, myoclonus or myoclonus ataxia, parkinsonism, and autoimmune movement disorders. With a wide range of neurological symptoms, clinicians should be well aware of the toxic profile of Hg and detailed occupational, environmental, and drug history should always be taken.
Chelation with DMPS or its analogues has shown efficacy as therapeutic agents for Hg toxicity but more clinical trials are needed. Unfortunately, irreversible neuronal damage often already sets in by the time neurological signs manifest, thus chelation therapy may be ineffective. Thus, it is of utmost importance to eliminate the potential dietary or occupational source to prevent neurodegeneration. Finally, implementation and monitoring of strict regulatory guidelines and strategies to combat environmental Hg toxicity are indispensable to prevent its neurotoxicity and protect our beautiful world.
Take Home Message
-
1. Human exposure to Hg is still prevalent in the modern world.
-
2. Cerebellar ataxia, tremor, and myoclonus are common movement disorders from exposure to Hg.
-
3. Parkinsonism and autoimmune Morvan syndrome can be associated withHg.
-
4. Detailed occupational, environmental, and drug history are the keys to diagnose Hg-induced movement disorders.
-
5. Preventive strategies must be implemented globally to get rid of the toxic effects of Hg.
Conflict of Interest
No authors have any conflict of interest to disclose.
Statement of Authorship
1. Research project: A. Conception, B. Organization, C. Execution.
2. Manuscript Preparation: A. Writing of the first draft, B. Review and Critique.
JG: 1B, 1C, 2A.
DK: 1B, 1C.
MJ: 1A, 2B.







