Neuromyelitis optica is a common immunologic cause of visual and spinal symptoms in young adults. It is usually treated with corticosteroids and immunosuppression. Various differentials such as infections, malignancy such as lymphoma should be considered along with inflammatory demyelinating conditions while evaluating the etiologic cause of opticospinal syndromes as per Wingerchuk criteriaReference Wingerchuk, Banwell and Bennett1 for diagnosis of neuromyelitis optica spectrum disorders (NMOSD).
A 24-year-old woman from a village in south India presented with a 5-month history of weakness of both lower limbs with complete sensory loss below the level of the umbilicus associated with increased frequency of urination with an incomplete evacuation of the bladder which developed over a period of 1 month. There were no constitutional symptoms. Around 4 months after onset of lower limb weakness, she developed blurring of vision in both eyes. The best-corrected visual acuity was 6/18 in each eye. There was no relative afferent pupillary defect. Fundus showed findings demonstrated in Figure 1a–d. A diagnosis of opticospinal syndrome was made. Differential diagnosis of NMOSD was considered including sarcoidosis, vasculitic disorders, Neuro-Behcet’s disease as well as infiltrative diseases such as lymphoma and chronic infections. She was evaluated with magnetic resonance imaging (MRI) of the brain and spine which revealed longitudinally extensive transverse myelitis (LETM) with periventricular hyperintensities on fluid attenuation inversion recovery (FLAIR) images and fulfilled the imaging criteria for NMOSD by Wingerchuk et al. (Figure 2). However, there were red flags.
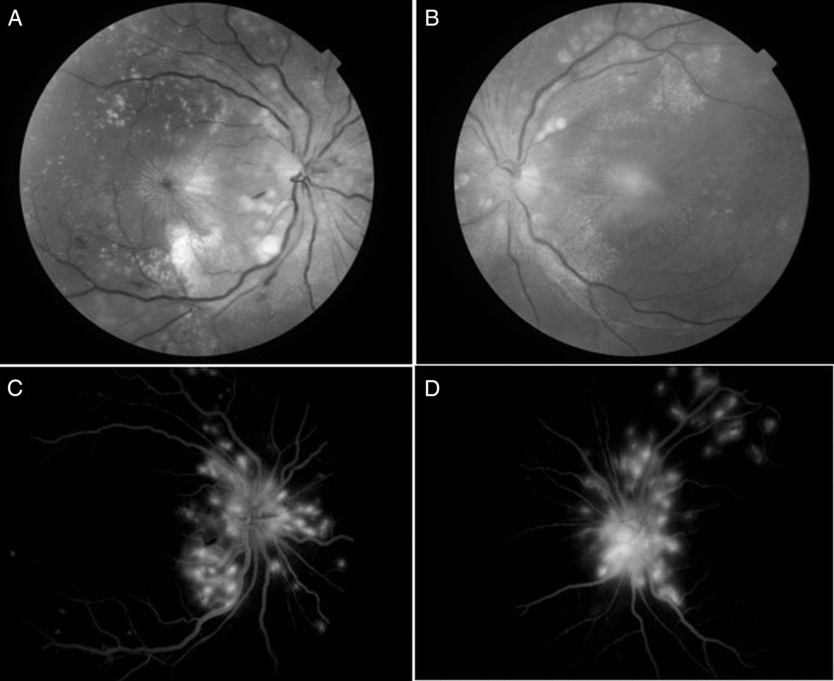
Figure 1: Fundus examination showed hyperemic disc edema with dilated, tortuous veins, retinal exudates, few streak hemorrhages, multiple choroidal tubercles and macular striae (A and B). Fundus fluorescein angiography (C and D) in late venous phase showed disc leakage with dilated tortuous veins and hyperfluorescent areas indicating active choroidal lesions. Presence of macular star is consistent with an infectious process, and is strongly against the diagnosis of inflammatory demyelinating illness
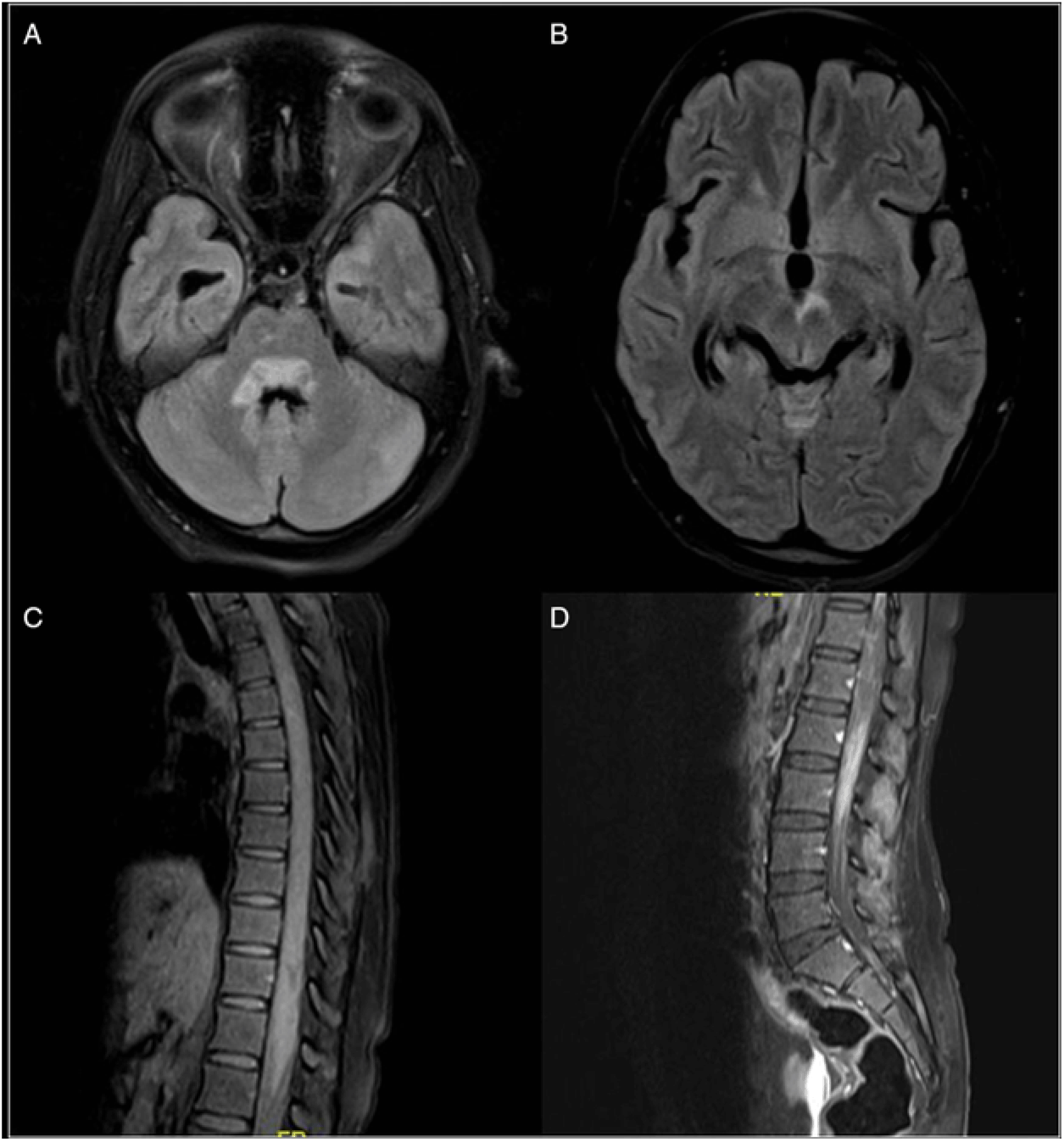
Figure 2: MRI brain (Panel A and B) T2/FLAIR hyperintensity in the periventricular regions around the third and fourth ventricle areas. She also had long segment spinal cord hyperintensity (involving more than 3 segments of the spinal cord) with patchy contrast enhancement (Panel C and D). There were no lesions of the optic nerve or chiasma, periventricular, juxtacortical or cortical lesions. [These MRI findings were consistent with a diagnosis of NMOSD, based on Wingerchuk et al. 2015 criteria.1 However, the other clinical findings of the fundus, CSF examination, disease course and progression as well as response to anti-tubercular therapy differentiated this case from NMOSD.]
Anti-aquaporin antibodies were negative. CSF examination revealed lymphocytic pleocytosis (1800 cells/mm3), raised protein (163 mg/dL) and normal sugar (72 mg/dL) with a corresponding blood sugar of 110 mg/dL and raised adenosine deaminase (12 U/L, normal <5 U/L) which were consistent with a diagnosis of tuberculosis. Acid fast bacilli and GeneXpert for rifampin were not detected in vitreous as well as CSF. HIV serology and VDRL were negative. Based on characteristic fundus findings, treatment with anti-tubercular medications and prednisolone was commenced. Myelopathy did not improve, however, CSF became acellular with no new deficits.
Tuberculosis can cause papillitis and choroiditis as well as myelitis mimicking NMO. Careful dilated fundus examination may show pathognomonic findings ensuring early diagnosis and appropriate treatment.
NMOSD have been defined using the Wingerchuk criteria. According to these criteria, if a patient has one episode of optic neuritis and myelitis even without anti-aquaporin antibody, a diagnosis of NMOSD can be made. However, it is very important to remember that other conditions can mimic neuromyelitis optica and they should be ruled out before NMOSD are diagnosed. Infectious causes such as tuberculosis can cause myelopathy as well as vision loss due to choroiditis, optic nerve infiltration, drug-induced optic neuropathy or hydrocephalus-related intracranial hypertension and secondary optic atrophy.Reference Garg, Malhotra, Kumar and Uniyal2 It is critical to differentiate this because the treatment of NMOSD is immunosuppression, which could exacerbate the infection if it is missed while anti-tubercular therapy should be initiated without delay in tuberculosis.
Although the patient had visual impairment and myelopathy and imaging was consistent with NMOSD, the other Wingerchuk criteria for NMOSD with negative anti-AQP4 antibody were not fulfilled. Importantly, the patient had vision impairment which was not severe as seen in NMOSD and the pupils were briskly reacting to light. This is an important sign, as usually patients with significant optic neuritis present with absent pupillary light reflex and relative afferent pupillary defects. This patient had fundus findings and CSF features suggesting the alternate diagnosis of tubercular chorioretinitis with myelopathy.
Ocular tuberculosis develops due to hematogenous spread or as a hypersensitivity to a distant focus of infection. The most common ocular manifestation is choroidal tubercles. The diagnosis is challenging and most often it is presumed ocular tuberculosis based on the clinical findings, ancillary tests and therapeutic trial of anti-tuberculous treatment. Aqueous and vitreous tap or CSF analyses for TB-PCR or culture have low sensitivities.Reference Garg, Malhotra, Kumar and Uniyal2– Reference Gupta, Gupta and Rao4
The main types of choroidal involvement in tuberculosis include tubercles, large tuberculoma, choroiditis or subretinal abscess occurring due to necrosis within a granuloma. Choroidal tubercles are small gray-white to yellow nodules less than a quarter disc diameter, which are not well circumscribed. Neuroretinitis typically occurs from choroidal spread of infection in a peripapillary location.Reference Dalvin and Smith5 Active choroidal tubercles tend to show early hypo- and late hyper-fluorescence on fundus fluorescein angiography.Reference Bansal, Basu, Gupta, Rao, Invernizzi and Kramer6
Presumed ocular tuberculosis with central nervous system involvement has been reported in the setting of meningitis, meningoencephalitis and optochiasmatic arachnoiditis, but its association with myelitis is rare.
Choroidal tubercles are pathognomonic of tuberculosis. Presence of macular star in the fundus strongly favors infective rather than demyelinating illnesses. Macular star and choroidal tubercles in the same patient with tuberculous myelitis have not been reported so far.
In tropical countries, it is always important to rule out tuberculosis as the treatment used in the treatment of NMO can lead to flare of the infection and may be fatal.
Conflicts of Interest
The authors have no conflicts of interest to declare.




