INTRODUCTION
Background
Neuropathic pain has been defined as “pain caused by a lesion or disease of the somatosensory system.”Reference Jensen, Baron and Haanpaa 1 , Reference Treede, Jensen and Campbell 2 Specifically, central neuropathic pain develops from an injury to the central nervous system (CNS) (brain, brainstem, or spinal cord), and includes diverse etiologies such as trauma, infarction, demyelination, or neoplasia. The most common central neuropathic pain syndromes are a result of stroke, referred to as central post-stroke pain (CPSP), spinal cord injury (SCI), or multiple sclerosis (MS). These pain syndromes are much less common than peripheral etiologies, and consequently less is known regarding optimal treatment and long-term outcomes.
Few randomized controlled trials have been performed to assess the efficacy of pharmacotherapy for specific central neuropathic pain syndromes, and evidence can be conflicting.Reference Watson and Sandroni 3 In general, the pharmacological management of central and peripheral neuropathic pain is similar. Guidelines based on meta-analyses and expert consensus have recommended the use of gabapentinoids, serotonin-norepinephrine reuptake inhibitors and tricyclic antidepressants as first-line therapy.Reference Finnerup, Attal and Haroutounian 4 , Reference Moulin, Boulanger and Clark 5 Despite widespread prescription, opioid analgesics are considered second or third-line therapy, and their long-term use may be associated unfavorable clinical outcomes.Reference Moulin, Clark and Gordon 6 There is also increasing interest regarding the use of cannabinoids in treating neuropathic pain.Reference Meng, Johnston, Englesakis, Moulin and Bhatia 7
Objective
The literature regarding the long-term treatment of central neuropathic pain syndromes is limited, and lacks external validity that could be applied to real-world outcomes. Recently, a database has been established to prospectively evaluate the clinical effectiveness of neuropathic pain management in Canadian tertiary care centers.Reference Moulin, Clark and Gordon 6 , Reference Mai, Clark and Gordon 8 Utilizing these data, the objective of the current study is to specifically determine the real-world management of central neuropathic pain relative to peripheral neuropathic pain.
MATERIALS AND METHODS
Study Design and Outcome Measures
This clinical trial was designed based on guidelines established by the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials.Reference Dworkin, Turk and Farrar 9 Standard chronic pain outcome measures were obtained at baseline, 3, 6, and 12 months. The primary outcome measure was the composite of a reduction of ≥30% in average pain intensity and 1-point drop in the Pain Interference Scale of the Brief Pain Inventory (BPI; 0-10) relative to baseline at 12 months. Secondary outcome measures were impact on function (Pain Disability Index), mood (Profile of Mood States), quality of life (12-item short form health survey [SF-12]), catastrophizing (Pain Catastrophizing Scale), and patient satisfaction (Patient Global Satisfaction Scale). Patient use of prescription medications, including opioid dosages, was recorded at baseline and at 12-month follow-up.
Study Setting and Participants
The Canadian Neuropathic Pain Database has been established as a registry of patients with neuropathic pain syndromes referred to seven Academic Tertiary Pain Centers.Reference Moulin, Clark and Gordon 6 From this registry, a prospective observational cohort study identified patients with central (n=79) and peripheral neuropathic pain (n=710). Demographic variables collected from study participants included age, sex, pain duration, level of education, smoking status, analgesic and marijuana use, comorbidities, and disability compensation. The diagnosis of neuropathic pain was based on clinical criteria and supported by validated questionnaires (Douleur Neuropathique en 4 [DN4]).Reference Bouhassira, Attal and Alchaar 10 , Reference VanDenKerkhof, Stitt and Clark 11 Initial patient assessment and subsequent follow-ups were conducted in-person at each Pain Center. Study follow-up was arranged for 3, 6, and 12 months in all patients to assess the efficacy of treatment.
This study was approved by independent review boards representing each participating institution (University of Calgary, Alberta; Western University, McMaster University, University of Toronto and University of Ottawa, Ontario; McGill University, Quebec; Capital District Health Authority Research Ethics Board, Nova Scotia).
Statistical Methods
Between-group differences in baseline patient characteristics were made using χ 2 tests or Fisher’s exact test for categorical characteristics and unpaired t-tests for continuous characteristics except for pain duration, for which a Wilcoxon two-sample test was performed. Between-group comparisons regarding analgesic history were made using χ 2 tests and, for opioid dose, Wilcoxon two-sample tests. The means and standard deviations for continuous characteristics, and frequencies and percentages for categorical characteristics, were calculated for baseline and for 12-month follow-up. 95% confidence intervals (CIs) are included for the composite outcome. McNemar’s χ 2 test was used for dichotomous values to assess the change in the proportion of patients using major classes of analgesics (analgesic antidepressants, anticonvulsants, opioid analgesics) from baseline to the 12-month follow-up. The change in the opioid dose was evaluated using a signed-rank test.
Fisher’s exact test was used to detect any difference in responder rate (achievement of primary outcome) between those being treated with two analgesic classes and those being treated with all three classes. For secondary outcome measures, baseline and 12 month values were compared using paired t-tests. The p-value comparing secondary outcomes was obtained by using an unpaired t-test comparing the changes in the two groups (central vs. peripheral), equivalent to the interaction between time (baseline vs. follow-up) and group (peripheral vs central). Between-group comparisons were made using an unpaired t-test for the difference in primary outcome based on opioid doses.
Descriptive statistics include means and standard deviations or medians and quartiles for continuous variables and frequencies and percentages for categorical variables. Between-group comparisons of the central neuropathic pain subjects with the peripheral pain subjects were made using χ 2 tests, or Fisher’s exact tests where expected cell sizes were less than five, for dichotomous variables and unpaired t-tests, or Wilcoxon’s two-sample tests where data were not normally distributed, for continuous variables. To quantify the primary endpoint (composite of a reduction of ≥30% in average pain intensity and 1-point drop in the Pain Interference Scale of the BPI relative to baseline at 12 months), 95% CIs were calculated for the percentages within the two groups and for the percentage differences between the two groups. For secondary outcomes the 12-month change from baseline was calculated, along with the associated 95% CIs, were calculated and the between-group differences compared using unpaired t-tests.
McNemar’s χ 2 test was used to assess the change in the proportion of subjects using major classes of analgesics from baseline to the 12-month follow-up in the central neuropathic pain group while the change in the opioid dose was evaluated using a signed-rank test.
RESULTS
Participants and Descriptive Data
Figure 1 demonstrates patient flow through the study. A total of 789 patients fulfilled eligibility for recruitment into the Canadian Neuropathic Pain Database. Of these patients, 80 were identified as having central neuropathic pain, 79 of which were included in the current analysis (Table 1). One patient was excluded, as further review identified their pain as being peripheral in origin. The baseline characteristics and analgesic history of these 79 patients are outlined in Tables 2 and 3, respectively, with comparison to patients with peripheral neuropathic pain. Additional 14 patients were lost to follow-up (17.7%). The data from 65 patients were available for statistical evaluation, of which 52 patients had completed data sets at 12-month follow-up. The diagnostic details of the 710 patients with peripheral neuropathic pain were previously published.Reference Moulin, Clark and Gordon 6 Complete data sets were available for 463 of these patients.
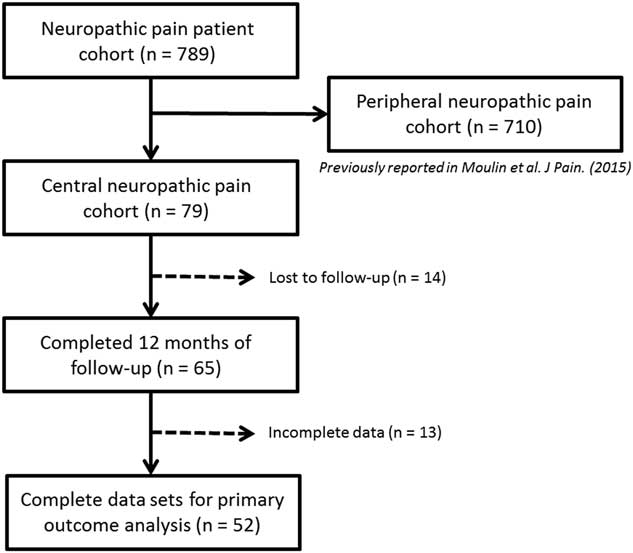
Figure 1 Flow diagram of study participants.
Table 1 Location and etiology of central neuropathic pain syndromes (n=79)
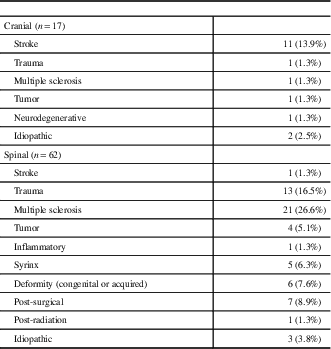
Table 2 Patient characteristics at baseline

DN4=Douleur Neuropathique en 4 Questions: score ≥4 indicates probable neuropathic pain; M=male.
Data are mean±standard deviation, median (Q1, Q3).
Table 3 Patient analgesic history
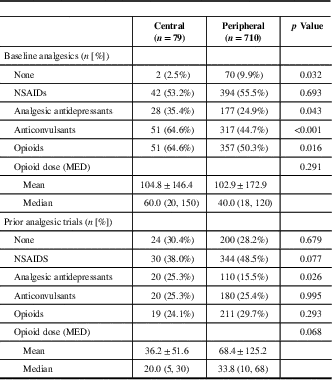
MED=morphine equivalent dose (mg/day); NSAIDS=nonsteroidal anti-inflammatory drugs.
Data are mean±standard deviation, median (Q1, Q3).
Outcome Data: Main Results
The proportion of patients achieving a ≥30% reduction in pain at 12 months relative to baseline was 7/52 or 13.5% (95% CI, 5.6-25.8), whereas those achieving a reduction of at least 1 point on the Pain Interference Scale was 20/52 or 38.5% (95% CI, 25.3-53.0) (Table 4). The proportion of patients reaching the primary outcome, including both a ≥30% reduction in pain and 1 point reduction on the Pain Interference Scale, was 5/52 or 9.6% (95% CI, 3.2-21.0). In comparison, patients with peripheral neuropathic pain syndromes were more likely to achieve this primary outcome at 12 months (25.3% of patients; 95% CI, 21.4-29.5) (difference [95% CI] of 15.7% [6.7%-24.6%], p=0.012).
Table 4 Primary endpoint and its components at month 12
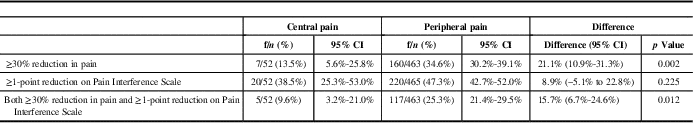
CI=confidence interval.
Outcome Data: Secondary Analyses
For secondary outcome measures, only mean pain intensity and mean Interference Scale Score were significantly improved at 12-month follow-up relative to baseline (p=0.031 and p=0.044, respectively). The additional outcome measures, including Profile of Moods State, SF-12 Mental, SF-12 Physical, Pain Disability Index, Pain Catastrophizing Scale and Patient Global Satisfaction did not achieve significance (Table 5). In comparison, patients with peripheral neuropathic pain achieved significant improvement in all secondary outcomes at 12-month follow-up relative to baseline (p<0.001). When comparing the two groups, patients with peripheral neuropathic pain were more likely to report improvements in Profile of Moods State, Pain Disability Index, and Pain Catastrophizing Scale (p=0.006, p=0.034, and p=0.030, respectively).
Table 5 Changes from month 0 to month 12 for secondary outcome measures

PMOS—SF=Profile of Mood States—Short Form; SF-12=12-item short form health survey.
BPI (0-10); PDI (0-70), higher score indicates greater disability; POMS-SF (0-120), higher score indicates greater impairment; SF-12 (0-100), score <50 indicates below average health status; Pain Catastrophizing Scale (0-52), higher score indicates greater distress; Pain Global Satisfaction (0-10), higher score indicates greater satisfaction.
The proportion of patients with central neuropathic pain using analgesic antidepressants, anticonvulsants, or opioids was unchanged at 12-month follow-up relative to baseline (Table 6). However, combination therapy was common with about 30% of patients receiving two of the three major classes of analgesics for neuropathic pain (antidepressants, anticonvulsants, and opioid analgesics) and about 17% treated with all three classes of analgesics concurrently at 12-month follow-up.Reference Moulin, Clark and Gordon 6 Opioids were routinely used by 37/65 (56.9%) of patients at 12-month follow-up, which represented a non-statistically significant decrease from 43/65 (66.2%) of patients at baseline. In total, 31 patients used opioids at both baseline and in follow-up, without any significant change in dosage (Table 7).
Table 6 Major analgesic class use at baseline and 12-month follow-up (n=65)
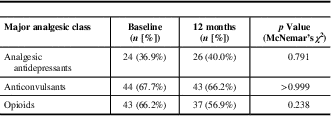
Analgesic antidepressants refer to tricyclic antidepressants and serotonergic noradrenergic reuptake inhibitors; anticonvulsants refer primarily to gabapentin and pregabalin.
Table 7 Opioid dose (morphine equivalent dose mg/day) in patients with opioid use at baseline and 12-month follow-up (n=31)

A number of non-pharmacological treatment modalities were commonly employed by patients with central neuropathic pain. The most commonly used by 12 months were physiotherapy and acupuncture, reported by 56.9% and 40.0% of patients, respectively. Local anesthetic or steroid injections were utilized by 24.6% of patients. Other methods included transcutaneous electrical nerve stimulation (20.0%), psychotherapy (15.4%), surgery (13.9%), and local anesthetic infusions (10.8%).
DISCUSSION
The primary outcome measure for this study was the proportion of patients achieving both at least a 30% reduction in average pain intensity and a 1-point reduction in the Interference Scale Score of the BPI at 12 months.Reference Dworkin, Turk and McDermott 12 These were chosen as they represent a clinically significant improvement in both pain and function. Only 9.6% of patients with central neuropathic pain achieved this outcome compared with 25.3% of patients with peripheral neuropathic pain. Interestingly, more patients with central neuropathic pain achieved a functional improvement (38.5%) compared with pain reduction (13.5%). The overall low success rate may be attributed to several factors, including the lack of specific therapeutic agents available to treat central neuropathic pain syndromes. The median pain duration was 4.0 years, suggesting that pain control had been refractory to conventional treatments initiated in a primary care setting; in addition, between 24.1% and 38% of patients had previously tried different analgesic classes and combination therapy was common among the entire cohort during the study. Furthermore, conducting this study at tertiary care centers is more likely to introduce a referral bias, with more complex patients being included in the analysis.
Diagnosing and distinguishing central neuropathic pain from other pain etiologies is essential to facilitate optimal care. Patients with neuropathic pain often suffer from comorbid musculoskeletal nociceptive pain and spasticity secondary to limb disuse and impaired mobility.Reference Watson and Sandroni 3 Allodynia and hyperalgesia, which reflect aberrant pain signaling, can be general features of both central and peripheral etiologies. Other pain characteristics allow further discrimination, including the temporal relation to a CNS injury, and distribution of pain within the area affected by the injury.Reference Watson and Sandroni 3 Validated outcome measures, such as the DN4 Questions, allows for screening of central neuropathic pain syndromes with high sensitivity.Reference VanDenKerkhof, Stitt and Clark 11
The etiologies resulting in central neuropathic pain syndromes are heterogeneous, and most commonly include CPSP, SCI, and MS.Reference Widerstrom-Noga, Loeser, Jensen and Finnerup 13 Accordingly, these three etiologies represented 47/79 or 59.5% of patients included in the current analysis. However, any lesion of the CNS can be implicated in the development of central neuropathic pain, including demyelination, tumors, trauma, syringomyelia, and sequela of surgery or radiation. Central neuropathic pain syndromes are distinctly less common than peripheral neuropathic pain, and as such our understanding is limited regarding the natural history of these disorders, optimal treatment paradigms, and long-term outcomes.Reference Watson and Sandroni 3 To our knowledge, the observations of the current study are the first to prospectively evaluate 12-month real-world treatment outcomes of a variety of central neuropathic pain etiologies. Patients with central neuropathic pain rarely achieve significant long-term benefit and are significantly less likely to respond to treatment than patients with peripheral neuropathic pain.
Neuropathic pain is associated with a lower health-related quality of life and suboptimal patient-reported outcomes.Reference Doth, Hansson, Jensen and Taylor 14 A previous cross-sectional study of community-based practices in the United States reported associations between severe neuropathic pain and poor function, less sleep, and increased anxiety and depression.Reference Schaefer, Mann and Sadosky 15 Although both peripheral and central neuropathic pain reported similar disability, only central neuropathic pain related to SCI was included in this analysis. The natural history of central neuropathic pain is not known, although studies of patients with chronic pain related to SCIReference Stormer, Gerner and Gruninger 16 , Reference Finnerup, Norrbrink and Trok 17 and MSReference Young, Amatya, Galea and Khan 18 demonstrate that pain tends to worsen over time and patients become more disabled. These poor outcomes highlight the need for effective treatment paradigms; however, there is a distinct lack of prospective controlled clinical trials for central neuropathic pain. Furthermore, previous studies have often reported conflicting evidence, with specific pharmacological agents useful in one central neuropathic pain syndrome but not another. For example, pregabalin has been demonstrated to provide significant pain relief in patients with neuropathic pain secondary to SCI,Reference Siddall, Cousins, Otte, Griesing, Chambers and Murphy 19 , Reference Vranken, Dijkgraaf, Kruis, van der Vegt, Hollmann and Heesen 20 but not CPSP.Reference Kim, Bashford, Murphy, Martin, Dror and Cheung 21 Gabapentin, a cornerstone in most neuropathic pain treatment paradigms, has mixed evidence regarding its effectiveness in SCI.Reference Levendoglu, Ogun, Ozerbil, Ogun and Ugurlu 22 , Reference Rintala, Holmes, Courtade, Fiess, Tastard and Loubser 23 Amitriptyline can be effective for CPSP,Reference Leijon and Boivie 24 and for SCI patients with comorbid depression.Reference Rintala, Holmes, Courtade, Fiess, Tastard and Loubser 23 Lamotrigine has generally been shown to be effective in treating both CPSP and SCI.Reference Vestergaard, Andersen, Gottrup, Kristensen and Jensen 25 , Reference Finnerup, Sindrup, Bach, Johannesen and Jensen 26 Both duloxetineReference Vollmer, Robinson, Risser and Malcolm 27 and cannabinoidsReference Rog, Nurmikko, Friede and Young 28 may be effective for MS-related pain.
Such disparities also exist in the treatment of other peripheral neuropathic pain syndromes,Reference Moulin, Boulanger and Clark 5 highlighting the need for an individualized approach to pharmacotherapy with different monotherapies or combination therapies.Reference Chaparro, Wiffen, Moore and Gilron 29 , Reference Gilron, Jensen and Dickenson 30 Given the limited data available and relative rarity of central neuropathic pain syndromes, the treatment approach to central neuropathic pain is often based on algorithms established for peripheral neuropathic pain.Reference Watson and Sandroni 3 Previous meta-analyses have reported that there is no general evidence for the use of a specific drug to treat a specific pain disorder, and thus pharmacotherapy guidelines are applicable to generalized neuropathic pain.Reference Finnerup, Attal and Haroutounian 4 , Reference Finnerup, Sindrup and Jensen 31 In general, first-line therapy consists of gabapentinoids, serotonin-norepinephrine reuptake inhibitors, and tricyclic antidepressants.Reference Moulin, Boulanger and Clark 5 Cannabinoids have recently been reported to have analgesic benefit in neuropathic pain,Reference Meng, Johnston, Englesakis, Moulin and Bhatia 7 , Reference Lynch and Campbell 32 although their use should not be considered first-line and larger studies are required to determine appropriate indications, dosing, and efficacy.
The role of opioid analgesics in the treatment of neuropathic pain is controversial. Although previously considered first-line treatment in many paradigms,Reference Dworkin, Backonja and Rowbotham 33 the current use of opioids is recommended as second or third-line therapy.Reference Finnerup, Attal and Haroutounian 4 , Reference Moulin, Clark and Gordon 6 This change has been prompted by concerns regarding the potential risks of abuse and opioid-related morbidity and mortality.Reference Edlund, Martin, Russo, DeVries, Braden and Sullivan 34 - Reference Imtiaz, Shield, Fischer and Rehm 36 The efficacy of strong opioids has been described (oxycodone, morphine), primarily in peripheral neuropathic pain, with maximum effectiveness achieved at 180 mg of morphine or morphine equivalent per day.Reference Finnerup, Attal and Haroutounian 4 In the current study, there was a small but non-significant decrease in the number of patients using opioids at baseline and 12-month follow-up, which may have reflected optimization of other adjunct therapies. A total of 31 patients with central neuropathic pain used opioids throughout the study, without any significant change in opioid dose. Results reported from the Canadian Neuropathic Pain Database demonstrate that patients with peripheral neuropathic pain and favorable outcomes at 12-month follow-up were less likely to be on opioids, or used significantly lower doses.Reference Moulin, Clark and Gordon 6 However, this was not the case for patients with central neuropathic pain whose outcomes were less favorable than patients with peripheral pain.
It is not clear why central neuropathic pain was managed less effectively than peripheral neuropathic pain. Patients with peripheral pain were more likely to achieve significant pain reduction, and also reported improved secondary outcome measures including pain disability and catastrophizing. These differences cannot be explained by differences in baseline patient characteristics. Although patients with central neuropathic pain reported were more frequently male, had longer pain duration, higher usage of antidepressants, anticonvulsants, opioids (although not higher opioid doses), and marijuana use than patients with peripheral pain, pain intensity, and disability were similar at baseline. The difference is likely more complicated and related to the underlying pathophysiology of central neuropathic pain, which is poorly understood.
A primary tenet of peripheral neuropathic pain is the activation of nociceptive pathways, with resultant aberrant signaling to various pain centers in the absence of ongoing noxious stimuli. Tissue injury or inflammation alters the local chemical environment of nociceptors, which upregulates inflammatory markers and induces downstream changes that potentiate receptor signaling.Reference Hucho and Levine 37 Central sensitization then refers to intrinsic changes in pain transmission pathways, particularly in the dorsal horn of the spinal cord, which potentiates pain signals.Reference Campbell and Meyer 38 In contrast, central neuropathic pain is a direct result of a CNS injury, and thus does not involve the same central sensitization mechanisms, which are sequelae of chronic pain.Reference Watson and Sandroni 3 Aberrant signaling within the spinal-thalamic-cortical pathways has commonly been implicated in the development of central neuropathic pain, although not all patients with spinothalamic tract lesions develop central neuropathic pain.Reference Andersen, Vestergaard, Ingeman-Nielsen and Jensen 39 It has been suggested that additional cofactors may be involved; regarding SCI for example, neuronal hyperexcitability within preserved spinothalamic tract neurons may be stimulated either directly via microglial activation or indirectly through disinhibition.Reference Zeilig, Enosh, Rubin-Asher, Lehr and Defrin 40 Abnormal, spontaneous burst activity within the thalamus has been implicated in a number of central neuropathic pain syndromes, suggesting a common central origin or amplifier.Reference Watson and Sandroni 3 As such, the relative poor effectiveness of pharmacotherapy in treating central neuropathic pain may thus be attributed to a number of factors, such as our limited understanding of which neurotransmitter(s) or signaling pathways should be targeted. In addition, central neuropathic pain may be intrinsically less responsive to treatment as it represents a true CNS injury, whereas peripheral pain syndromes are “stimulus evoked” and require potentiation with sensitization mechanisms,Reference Bennett 41 which tend to be clinically recognized earlier and thus can be inhibited or altered with medications.
Given the low success rate of treating central neuropathic syndromes, there has been renewed interest in the role of neuromodulation surgery. Unlike ablative procedures, neuromodulation is both titratable and reversible. Spinal cord stimulation is perhaps the most well-known and is commonly utilized for the treatment of failed back surgery syndrome and complex regional pain syndrome,Reference Krames 42 and its use in CPSP has been described with varying efficacy.Reference Dworkin, O’Connor and Kent 43 , Reference Aly, Saitoh, Hosomi, Oshino, Kishima and Yoshimine 44 The use of deep brain stimulation has a long-standing history in the treatment of pain, with some of the earliest indications being CPSP, facial anesthesia dolorosa, and phantom limb pain,Reference Hosobuchi, Adams and Rutkin 45 , Reference Mazars, Merienne and Cioloca 46 although pain invariably recurs and becomes refractory to stimulation.Reference Boccard, Pereira, Moir, Aziz and Green 47 Motor cortex stimulation has some reported efficacy in the treatment of CPSP and trigeminal neuropathic pain,Reference Tsubokawa, Katayama, Yamamoto, Hirayama and Koyama 48 , Reference Henderson, Lad and Chao 49 although long-term pain relief is inconsistent and tends to require intensive reprogramming.Reference Henderson, Boongird, Rosenow, LaPresto and Rezai 50 , Reference Sachs, Babu, Su, Miller and Henderson 51 Intrathecal drug delivery has an established history in the treatment of cancer-related pain and spasticity, with more recent interest in the use of intrathecal opioids and local anesthetics for the treatment of neuropathic pain syndromes.Reference Deer, Pope and Hayek 52 These procedures generally remain underutilized in the treatment of central neuropathic pain syndromes, although may potentially be considered as alternative therapies for refractory or intractable pain.
There are several limitations to this observational study. The primary issue is the small number of patients recruited with central neuropathic pain and the 17.7% loss to follow-up. However, this reflects the relative rarity of central neuropathic pain syndromes in the general population, and the low number of patients referred to tertiary care centers. Second, the relative heterogeneity of central neuropathic pain etiologies included in this analysis does not allow for the evaluation of specific interventions or conditions. Finally, as this was an observational study, there was no control group comprised of untreated patients. Despite these limitations, this study highlights the challenges in treating central neuropathic pain syndromes, and is the first to provide real-world evidence that central neuropathic pain is not managed as well as peripheral pain.
Acknowledgments
This study was funded by Canadian Foundation for Innovation (Grant #7878), The Earl Russell Chair in Pain Research, Western University, London, Ontario, and by Pfizer Canada.
Statement of Authorship
MDS was the primary investigator and was responsible for designing the article, interpreting and analyzing the data, and writing the manuscript. AJC, ASG, MEL, PKM-F, HN, CS, CT, MAW, and DEM assisted with the study design and revising of the article. LWS performed the statistical analysis and revised the manuscript for important intellectual content. All of the authors approved the final version of the manuscript submitted for publication and have agreed to act as guarantors of the work.
Disclosures
MDS, ASG, PKM-F, HN, LWS, and CT have nothing to disclose. AJC reports personal fees from Wex Pharmaceuticals, outside the submitted work. MEL reports other from Cannimed, other from CCIC, other from Panag Pharm Inc., outside the submitted work. In addition, MEL has a patent Cannabinoid based formulations for ocular pain issued. CS reports grants from Pfizer Canada, from null, during the conduct of the study; personal fees from Indivior, outside the submitted work. MAW reports personal fees from CHI, grants from CanniMed, outside the submitted work. DEM reports grants from Pfizer Canada, from null, during the conduct of the study.










