A 23-year-old Caucasian woman with 1 week of left-sided headache was admitted to our hospital with six stereotyped episodes of right-sided numbness, right-sided facial twitching, and expressive language difficulty lasting for 1-2 min. Past medical history included anxiety and a chronic (>10 years), stable right-sided neck mass that was biopsied and revealed only nonspecific inflammation with no evidence of infection or malignancy. Brain magnetic resonance imaging (MRI) with gadolinium performed on admission showed subtle left frontoparietal T2-fluid-attenuated inversion recovery (FLAIR) cortical hyperintensity with overlying leptomeningeal enhancement (Figure 1A and B), raising concern for focal meningoencephalitis. No abnormality was seen on T2-weighted or diffusion-weighted imaging. Electroencephalography performed inter-ictally showed left temporal slowing without epileptiform activity. Serologic testing for human immunodeficiency virus, syphilis, and anti-nuclear antibodies were negative. Computed tomography of the thorax/abdomen/pelvis and gallium scan showed no evidence of malignancy or sarcoidosis. Lumbar puncture on admission day 2 revealed a normal opening pressure, cerebrospinal fluid (CSF) lymphocytic pleocytosis (88 nucleated cells, 74% lymphocytes), elevated protein of 433 mg/L (200-400 mg/L), normal glucose, normal CSF/serum IgG index, and no CSF-specific oligoclonal bands. Upon recognizing in retrospect that her clinico-radiographic presentation was typical of unilateral cortical FLAIR-hyperintense Lesions in Anti-MOG-associated Encephalitis with Seizures (FLAMES), testing for myelin oligodendrocyte glycoprotein (MOG)-IgG was performed on stored CSF and was positive (fixed cell-based assay, EUROIMMUN). Comprehensive CSF testing for other antibodies associated with encephalitis, including N-methyl-D-aspartate receptor-IgG, was negative. No stored serum was available for MOG-IgG testing. She was prescribed lacosamide 100 mg PO BID without immunotherapy. One-month follow-up brain MRI showed resolution of her left frontoparietal abnormalities (Figure 1C and D), although a new, enhancing lesion of the corpus callosum compatible with demyelinating disease was seen (Figure 2). Cervicothoracic MRI was normal. She was monitored closely. Repeat brain MRI 8 months later was normal, and serum MOG-IgG testing 4 months and 8 months after disease presentation was negative. Lumbar puncture for repeat CSF MOG-IgG testing was not performed. At 12-month follow-up lacosamide was tapered off over 4 weeks, and she remains symptom-free at 15-month follow-up.
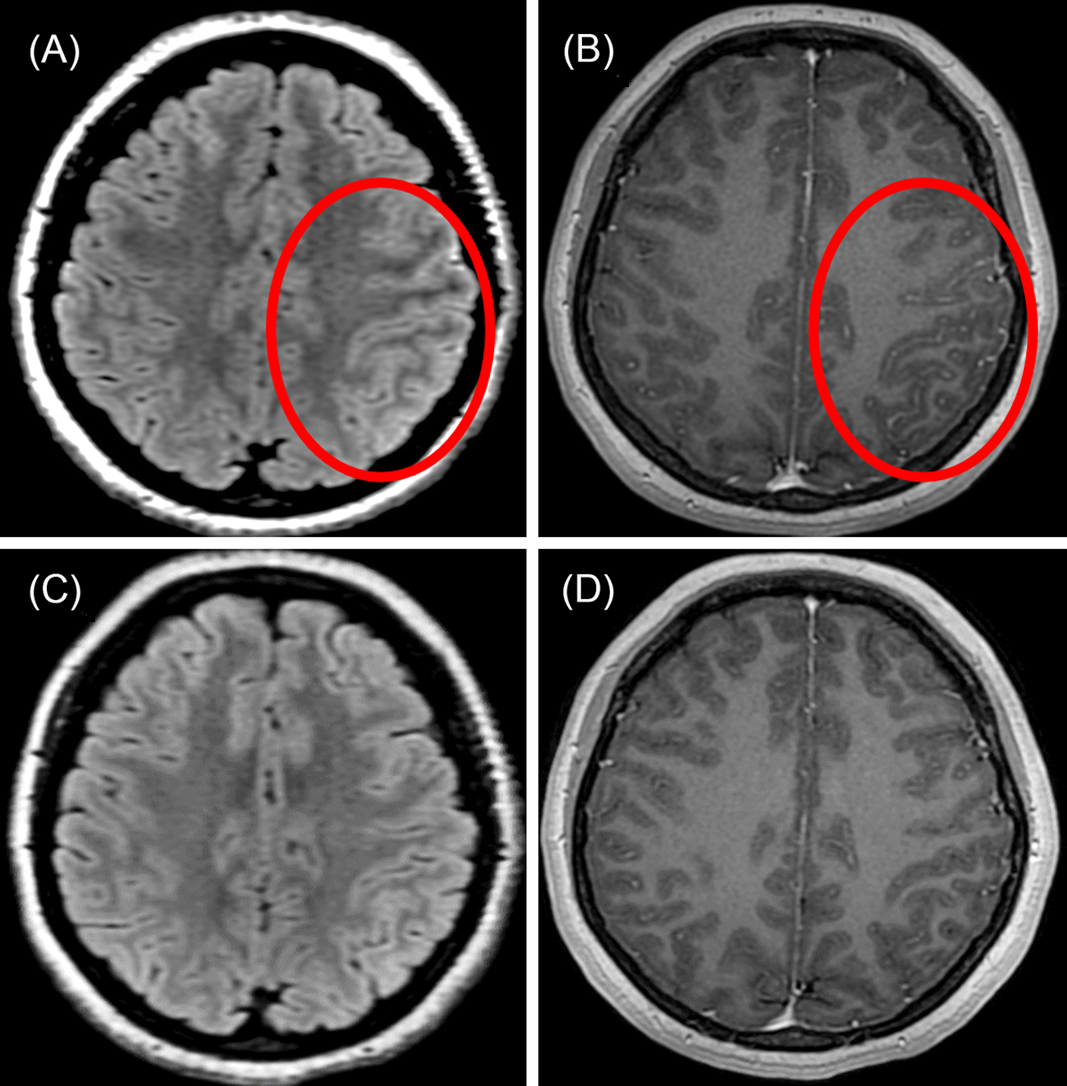
Figure 1: Brain MRI in unilateral cortical FLAMES. (A) Axial T2-FLAIR image shows subtle left frontal cortical hyperintensity (red circle). (B) Axial T1-weighted post-gadolinium image shows overlying leptomeningeal enhancement (red circle). (C and D) One-month follow-up imaging shows resolution of cortical and leptomeningeal abnormalities.

Figure 2: Brain MRI showing corpus callosum lesion after unilateral cortical FLAMES. (A) Sagittal T2-FLAIR image shows a small lesion of the genu of the corpus callosum (red arrow). (B) Sagittal T1-weighted post-gadolinium image shows lesion enhancement (red arrow).
MOG-IgG-associated disease (MOGAD) is a subset of inflammatory demyelinating disease that most commonly presents with optic neuritis, acute disseminated encephalomyelitis, transverse myelitis, or isolated brainstem syndromes Reference Jurynczyk, Messina and Woodhall1 . Unilateral cortical FLAMES is another recently characterized manifestation of MOGAD that is defined as follows: unilateral cortical T2-FLAIR hyperintensity without the involvement of the adjacent juxtacortical white matter on MRI, some combination of seizures, headache, fever, and/or cortical symptoms (e.g. aphasia) referable to the location of T2-FLAIR hyperintensity, MOG-IgG positivity by cell-based assay, and exclusion of alternative diagnoses. Reference Budhram, Mirian, Le, Hosseini-Moghaddam, Sharma and Nicolle2 Serum MOG-IgG testing is preferred due to generally lower clinical sensitivity (i.e. higher false-negative rate) of CSF, although fortunately, the available literature does not indicate lower clinical specificity (i.e. higher false-positive rate) of CSF that would cast doubt on the diagnosis in our patient Reference Mariotto, Alberto and Lucia3 . Nonetheless, lack of serum MOG-IgG testing at disease onset due to delayed recognition of unilateral cortical FLAMES is a limitation to our report that highlights the need for increased awareness of this unique MOGAD phenotype. Disease processes that may have a similar cortical and/or leptomeningeal neuroimaging appearance include Creutzfeldt–Jakob disease, seizure-related change, anti-gamma-aminobutyric acid (A) receptor encephalitis, mitochondrial encephalopathy with lactic acidosis and stroke-like episodes (MELAS), stroke-like migraine attacks after radiation therapy (SMART), reperfusion injury, subarachnoid hemorrhage, infectious and carcinomatous meningitides. Reference Renard, Castelnovo and Bouly4 Bilateral cortical and/or leptomeningeal involvement may uncommonly occur in MOGAD, Reference Budhram, Mirian, Le, Hosseini-Moghaddam, Sharma and Nicolle2 suggesting that unilateral cortical FLAMES exists on a broader spectrum of MOG-IgG-positive meningoencephalitis. Patients typically respond well to corticosteroids, although resolution without immunotherapy similar to our case has been reported. Reference Budhram, Sechi, Nguyen, Lopez-Chiriboga and Flanagan5 Recognition of this distinct clinico-radiographic syndrome is important for accurate diagnosis and treatment.
Conflicts of Interest
Dr. Mirian has nothing to disclose.
Dr. Yu has nothing to disclose.
Dr. Casserly reports personal fees from Roche, grants and personal fees from Biogen, personal fees from EMD Sorono, personal fees and others from Sanofi Genzyme, personal fees and others from Alexion, outside the submitted work.
Dr. Budhram has nothing to disclose.
Statement of Authorship
AM: literature review, drafting of the manuscript.
YY: critical revision of the manuscript for intellectual content.
CC: critical revision of the manuscript for intellectual content.
AB: design and conceptualization, critical revision of the manuscript for intellectual content.
The authors have not published, posted, or submitted any related manuscripts from the same study.




