Introduction
Leptomeningeal collaterals connect distal arterioles of the major cerebral arteries. In the event of an arterial occlusion or flow-limiting stenosis, they provide alternative routes for brain perfusion. Reference Liebeskind1 Flow through collaterals is associated with perfusion of ischemic brain and good functional outcome following acute ischemic stroke in patients treated with reperfusion therapies. Reference Menon, Smith and Modi2–Reference Nannoni, Sirimarco and Cereda4
Variability in collaterals is explained by both modifiable and non-modifiable cardiovascular risk factors, furthermore, rodent models have demonstrated that genetic differences may influence variability in collaterals. Reference Zhang, Prabhakar, Sealock and Faber5–Reference Lucitti, Sealock and Buckley7 Patient comorbidities and laboratory findings such as hypertension, congestive heart failure, older age, metabolic syndrome, hyperuricemia, elevated creatinine levels, and hyperglycemia have been variably reported as being associated with worse collaterals. Reference Liebeskind, Tomsick and Foster3,Reference Nannoni, Sirimarco and Cereda4,Reference Malik, Hou and Vagal8,Reference Menon, Smith and Coutts9 Whether genetic differences modulate variance in collateral status through these clinically observable factors, or whether they are modulated through independent genetic mechanisms is unknown. Prior researchers have sought to elucidate associations between clinically observable factors and collateral status, however the contributions of these factors to overall collateral variability is unknown.
Using a large cohort of ischemic stroke patients enrolled in the Measuring Collaterals with Multi-phase CT Angiography in Patients with Ischemic Stroke (PRove-IT) study, we examined the relative contribution of various demographic, laboratory, and clinical factors to between-patient variability in collateral status, and explored predictors of poor collaterals.
Methods
The PRove-IT study was a prospective multi-centre hospital-based cohort study that recruited patients with acute ischemic stroke and evidence of intracranial occlusion on computerized tomography angiography (CTA) presenting within 12 hours of symptom onset. We included patients from the PRove-IT study who were 18 years or older and had an acute ischemic stroke with an intracranial anterior circulation occlusion identified on multiphase CTA (mCTA). Patients with hemorrhagic or posterior circulation strokes, secondary occlusions reported on mCTA, inability to determine collateral score, and premorbid modified Rankin scale greater than 2 were excluded.
Collateral status refers to the robustness of the network of vascular channels that stabilize cerebral blood flow when principal conduits fails. Reference Liebeskind1 Collaterals were graded using mCTA images and the ColorViz tool (FastStroke, GE Healthcare, Milwaukee, Wisconsin). ColorViz incorporates all mCTA phases into a single time-variant color map of overall and phase-specific extent of pial enhancement; described in detail elsewhere. Reference Ospel, Volny and Qiu10,Reference Verdolotti, Pilato and Cottonaro11 The extent of collaterals for the primary occlusion were graded by switching the collateral display to a single-color mode and comparing the extent of vessels in the affected territory downstream of the occlusion to the corresponding vascular territory on the unaffected hemisphere. All images were assessed in a consensus reading by a stroke neurologist and neuroradiologist. Images were graded according to Tan’s classification, specifically, Tan Class i (absent collateral supply of affected territory), Tan Class ii (collateral supply filling >0% but ≤50% of affected territory), Tan Class iii (collateral supply filling >50% but <100% of affected territory), and Tan Class iv (100% collateral supply to affected territory). Reference Tan, Dillon, Liu, Adler, Smith and Wintermark12 In our statistical analyses, patients were classified as having either poor (<50%; Tan Classes i and ii), good (>50%; Tan Class iii), or optimal (100%; Tan Class iv) collaterals (Figure 1). We combined Tan Classes i and ii into a single "poor" collaterals group for our analyses because only 8 (2.1%) patients were classified as Tan Class i.
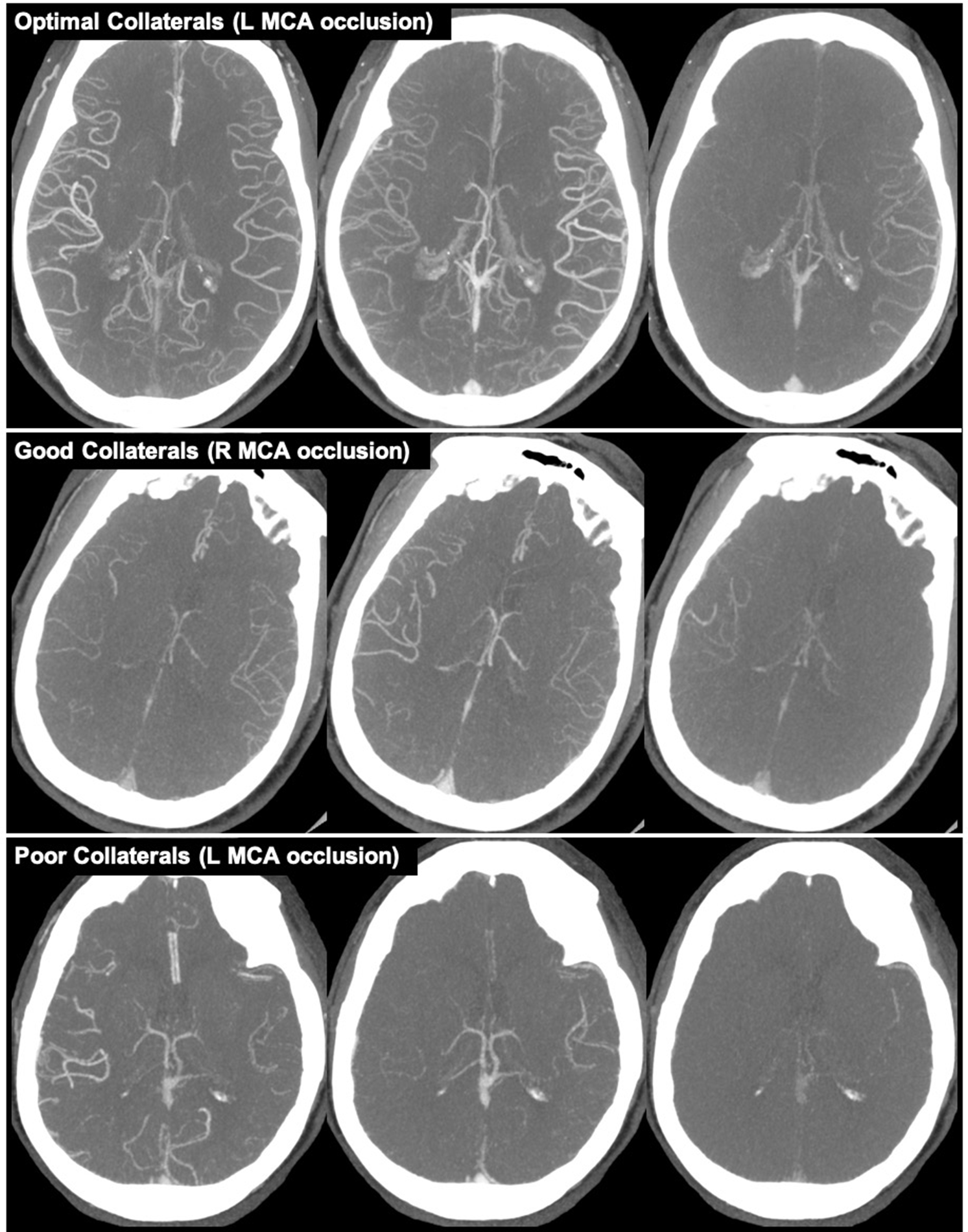
Figure 1: Example of collaterals grades based on Tan classification. Top row (left MCA occlusion): optimal collaterals (100%; Tan Class iv). Middle row (right MCA occlusion): good collaterals (>50%; Tan Class iii). Bottom row (left MCA occlusion): poor collateral (<50%; Tan Classes i and ii).
Between-group differences for demographic, laboratory, and clinical factors were compared using Pearson Chi-Square test for categorical variables and Mann-Whitney U or independent Student’s t-test for continuous variables. Association between demographic, laboratory, and clinical factors and collateral status were examined with ordinal regression models. Co-variables were selected for the multivariable model if they met significance at the p < 0.1 level in univariable between-group comparisons. Multivariable ordinal regression model building was done manually using backward elimination and forward selection, until parsimonious models were fit including only dependent variables considered significant at the two-sided p < 0.05 level. Reference Menon, Smith and Modi2 Interaction effects between predictor variables were explored in a post-hoc analysis.
Two logistic regression models were used to generate predicted probabilities of both poor and impaired collaterals. All measured demographic, clinical, and laboratory variables forced into the models as independent variables. In the first model, the dependent variable was collateral classification, either Poor (Tan Classes i-ii) or Good (Tan Classes iii-iv) collaterals. The outcome used to generate predicted probabilities was ‘Poor collaterals’ as defined by the modified Tan scale. Reference Yeo, Paliwal and Teoh13 In the second model, patients were classified as either Impaired (Tan Class i-iii) or Optimal (Tan Class iv) collaterals. The outcome used to generate predicted probabilities was ‘Impaired collaterals, defined as <100% collaterals in the affected territory. For each model, predicted probabilities of being in the group of interest (i.e. either Poor or Impaired collaterals) were generated for each participant. These predicted probabilities were then used to generate a linear regression against true outcomes (i.e. either Poor or Good collaterals for model 1; or either Impaired or Optimal collaterals for model 2) in order to calculate the r2, a measure of how much variability in the pre-specified outcome is explained by the dependent variables included in the model. This analysis was limited to patients without any missing data. If an individual’s health record did not include prior diagnoses, e.g. hypertension, it was assumed they were not present.
The experimental protocol was approved by local Research Ethics Boards and conformed to the Declaration of Helsinki. All data analyses were conducted in SPSS Statistics (version 23.0, IBM Corp., Armonk, NY).
Results
Of 614 patients, 386 met eligibility criteria (mean age 70.1 ± 12.5 years, 52.1% male) for our study. Collateral grades were distributed amongst Tan Class i (n = 8; 2.1%), Tan Class ii (n = 56; 14.5%), Tan Class iii (n = 125; 32.4%), and Tan Class iv (n = 197; 51.0%). The median time from symptom onset to CT was 120 (IQR: 78–246) minutes. There was no significant difference in symptom onset to CT between collateral groups (p = 0.16, Table 1). Occlusion sites were ICA (n = 60, 15.5%), M1 (n = 191, 49.5%), M2 (n = 78; 20.2%), M3 (n = 34, 8.8%), M4 (n = 13, 3.4%), A2 (n = 5, 1.3%), A3 (n = 3, 0.8%), and A4 (n = 2, 0.5%) segments. Treatment decisions included intravenous tissue plasminogen activator (tPA; n = 99, 25.6%), intra-arterial tPA (n = 84, 21.8%), intravenous and intra-arterial tPA (n = 136, 35.2%), antithrombotic therapy (n = 56, 14.5%), tenecteplase (n = 8, 2.1%), and no treatment (n = 3, 0.8%). There was no significant difference between collateral groups in treatment decision, p = 0.321. Data on mechanical thrombectomy was not available as part of PRove-IT.
Table 1: Demographic, laboratory and clinical outcomes comparing Poor (Tan Classes i and ii), Good (Tan Class iii), and Intact (Tan Class iv) collaterals on multiphase computed tomographic angiography.

* p < 0.05.
Significant between-group differences in age, sex distribution, leukocytes, total cholesterol, HDL, and fibrinogen were observed in univariable analysis (Table 1). Stroke etiologies included extracranial large artery atherosclerosis (n = 43, 11.1%), intracranial artery atherosclerosis (n = 12, 3.1%), cardioembolic (n = 208, 53.9%), embolic stroke of undetermined source (n = 94, 24.4%), other (n = 17, 4.4%), and missing (n = 12, 3.1%). There was no significant difference in stroke etiologies between collateral groups (p = 0.794; Table 2).
Table 2: Etiology of stroke by baseline collateral status, n (%). There is no significant difference in stroke etiology between collateral groups (p = 0.794).

In univariable ordinal regression analysis, male sex (p < 0.001), younger age (10 year increments; p = 0.013), elevated leukocytes (increments of 1 × 109/L; p = 0.017), and elevated fibrinogen (increments of 0.1 μmol/L; p = 0.023) were significant predictors of poorer collaterals. In multivariable ordinal regression modelling model, male sex (p = 0.005), younger age (10 year increments; p = 0.046), and elevated leukocytes (increments of 1 × 109/L; p < 0.001) were associated with poorer collaterals (Table 3).
Table 3: Ordinal regression univariable and multivariable models evaluating predictors of poor collateral status (Tan Classes i and ii).

CIs = confidence intervals; OR = odds ratio.
When exploring sex differences further, analysis showed that compared to females, males were more likely to have a history of coronary artery disease (23.9% v. 11.9%, p = 0.002) and smoking (51.2% v. 36.2%, p = 0.003; Supplemental Table S1). There was a non-significant trend showing that younger females were more likely to have poor collateral status when compared to older females (Supplemental Table S2). A significant multiplicative interaction (p < 0.001) between age and sex was found in post-hoc analysis. When this interaction variable was forced into the multivariable model, male sex was no longer a significant predictor of poor collaterals (p = 0.79).
Finally, all of our measured variables explained 44.8% (Impaired versus Optimal collaterals model) to 53.0% (Poor versus Good model) of the observed variance (r Reference Menon, Smith and Modi2 ) in collaterals. Thus, these models failed to explain 47.0–55.2% of the variance in collateral status.
Discussion
In this prospective multi-center hospital-based cohort of patients with anterior circulation ischemic strokes and good premorbid function, male sex, leukocytosis, and younger age were associated with worse collaterals. Comprehensive demographic, laboratory, and clinical variables explained 44.8–53.0% of between-patient variability in collaterals. This study provides further supportive evidence regarding variables that potentially explain differences in leptomeningeal collaterals in patients following acute ischemic stroke. Additionally, this study provides novel evidence that current measured (modifiable and non-modifiable) variables only explain half of the variability in collateral status between patients.
Rodent models have identified specific genes associated with leptomeningeal collateral diameter, density, and remodelling following occlusive events. Reference Zhang, Prabhakar, Sealock and Faber5–Reference Lucitti, Sealock and Buckley7,Reference Prabhakar, Zhang, Chen and Faber14–Reference Okyere, Giridhar and Hazy16 However, the relative contributions of genetic differences to collateral variability is unknown. To determine the amount of variance in collateral status explained by known and hypothesized predictors of collateral status, we built a model that included every measured demographic, laboratory, and clinical variable collected as part of the PRove-IT study. This model failed to account for 47.0–55.2% of the between-patient variability in collaterals. We therefore hypothesize that a large component of the unexplained variance may be due to genetic variance. It is also likely that other unmeasured non-genetic factors, including physiological variables such as cardiac and vascular endothelial function, contribute to this unexplained variability.
Our study provides some evidence for an association between male sex and poorer collaterals, however this effect of sex on collateral status is confounded by an interaction with age. Estrogen is protective in ischemic stroke via vasodilatory and anti-inflammatory effects. Reference Faber, Moore, Lucitti, Aghajanian and Zhang17,Reference Liu and McCullough18 Although most female subjects in this study were older than the median age of menopause, Reference Costanian, McCague and Tamim19 a hormone-independent mechanisms for reduced ischemia following hypoxic event suggesting sexual dimorphism in intrinsic cellular response may explain our observation. Reference Faber, Moore, Lucitti, Aghajanian and Zhang17 Furthermore, in our cohort males had higher smoking rates (51.2% vs. 36.2%) and were more likely to have a history coronary artery disease (23.9% vs 11.9%) – both markers of worse collateral status. Their aggregate effects may have contributed to the poorer collaterals observed in males. Reference Nannoni, Sirimarco and Cereda4,Reference Menon, Smith and Coutts9
We found an association between leukocytosis and poorer collaterals following anterior circulation ischemic stroke. It is plausible that leukocyte adherence to damaged endothelium, leading to microvascular occlusions, in addition to leukocyte mediated cytotoxic and inflammatory effects, result in poorer collaterals. Reference Danton and Dietrich20,Reference Iadecola and Anrather21 A reduction in collaterals may explain the association between leukocytosis and reduced cerebral blood flow following ischemic stroke. Reference Danton and Dietrich20,Reference Iadecola and Anrather21 This theory provides potential rationale for the worse outcomes and larger infarct volumes observed in patients with elevated early peripheral leukocyte counts following ischemic stroke. Reference Buck, Liebeskind and Saver22,Reference Liu, Wang and Shi23
Age-dependent collateral insufficiency has been well-established in rodent and human studies, and is thought to be due in part to dysfunctional endothelial nitric oxide synthase activity with aging. Reference Nannoni, Sirimarco and Cereda4,Reference Malik, Hou and Vagal8,Reference Menon, Smith and Coutts9,Reference Faber, Zhang and Lassance-Soares24–Reference Epstein, Lassance-Soares, Faber and Burnett26 In our cohort, we found a weak association between older age and better collaterals, which is discordant with the available literature and may reflect selection bias or confounding. All enrolling centers in the PRove-IT study were regional stroke referral centers. Therefore, patients considered for enrolment within the PRove-IT study, had to be clinically well-enough to be considered and accepted for urgent transfer to a stroke center. It is possible that older patients in our sample were biased towards those without comorbidities and better underlying collateral status, since they would be good candidates for thrombolysis or interventional therapy and would be accepted for transfer to a stroke center. Previously published clinical studies demonstrating older age to be associated with worse leptomeningeal were single-center studies enrolling all consecutive patients with acute ischemic stroke. Reference Nannoni, Sirimarco and Cereda4,Reference Menon, Smith and Coutts9 These prior studies likely captured a more representative acute ischemic stroke population.
An important clinical consideration when considering pial collaterals is the anatomic location of the occlusion. For example, carotid terminus occlusions limit potential collateral supply to the ipsilateral anterior cerebral artery only if there is adequate flow through the anterior communicating artery. If the anterior communicating artery is hypoplastic or absent collateral flow would be restricted. Given the objective of our study was to identify demographic, laboratory, and clinical variables that may contribute to pial collaterals, and their relative contribution to inter-patient variability in collaterals, we excluded patients with multiple occlusions. The presence of multiple occlusions would have confounded our analysis. When we assessed collateral status in our study, we determined the extent of collateral present within the anatomic territory that was at risk of collateral impairment, thereby controlling for anatomical variability between patients. Clinically, when determining which patients are at high risk of clinical deterioration prior to thrombolysis or thrombectomy, i.e. poor collaterals, it is imperative to closely examine the anatomic location of occlusion, the anatomy of any potential named arteries that may provide collateral supply, and for the presence of multiple occlusions.
This study has limitations. Although we analyzed most variables considered relevant in explaining collateral status variability, it is plausible that other non-measured variables contributed to variability in collateral status. Therefore, we are unable to parse with certainty the relative contribution of between-patient genetic differences versus unmeasured non-genetic differences in explaining collateral variability. The Tan collateral grading method is well-used within the neuroradiology literature; however, it has not been validated against digital subtraction angiography and its inter-observe reliability is unknown. We attempted to limit inter-observer differences in scoring by reading images in a consensus manner. In addition to intrinsic limitations of the Tan method, limitations in the correct identification of distal occlusions exist. Given this, we performed sensitivity analysis that excluded all distal exclusions (i.e. M3, M4, A3, A4). In this sensitivity analysis, within the multivariate model, sex and leukocytes remained significant predictors of collateral status, whereas age did not reach statistical significance (p = 0.076). In the univariate model, age, sex, and leukocytes remained significant predictors of collateral status, whereas fibrinogen did not (p = 0.367). The PRove-IT study did not collect data on menopausal status, therefore we are unable to extrapolate the complex interaction between estrogen exposure, age, and collateral status in female patients. There is a possibility that different anti-hypertensive classes have different effects on pial collaterals, for example calcium-channel blockers may preferentially affect pial collaterals when compared to diuretics. Within our cohort, 48.4% of patients using an anti-hypertensive were on multiple agents, which was too heterogeneous to explore whether different subclasses of anti-hypertensive varied between collateral groups. Furthermore, reported anti-hypertensive dosages, and dosages for all reported medications, were inconsistently collected across study sites. Given this, we grouped all anti-hypertensive subclasses together. In our cohort, intracranial atherosclerosis disease was the stroke etiology in only 3.1% of patients. Given that in some populations, atherosclerosis disease causes up to 50% of ischemic strokes, Reference Holmstedt, Turan and Chimowitz27 the significance of factors affecting pial collateral status in populations with a greater burden of atherosclerosis disease may be different.
Conclusion
Collateral status is an indicator of favorable outcome in stroke. We examined factors associated with collaterals and their relative contribution to between-patient variability in collaterals. Nearly half of the variance in collateral status remains unexplained and could be due in part to variability in genetics and other unmeasured non-genetic factors. Male sex and leukocytosis were associated with poorer collaterals. We did not find any modifiable factors to be associated with collateral status in acute ischemic stroke. Further research is needed to elucidate the relationship between genetics and collateral variability in humans.
Acknowledgements
The Prove-IT study was funded by the Canadian Institute of Health Research.
Conflict of Interest
Dr. Field receives in-kind study medication from Bayer Canada and has received a speaker’s bureau honorarium from Servier. She is supported by a Sauder/Heart and Stroke Professorship from the University of British Columbia, the Vancouver Coastal Health Research Institute, the Michael Smith Institute for Health Research, and the Heart and Stroke Foundation of Canada. Dr. Goyal reports grants from Medtronic, personal fees from Stryker, personal fees from Microvention, personal fees from Medtronic and personal fees from Mentice. In addition, Dr. Goyal has a patent to Systems of acute stroke diagnosis and licensed and a patent to Systems of intracranial access licensed. Dr. Menon has a patent to Systems of triage in acute stroke. Dr. Hill reports grants from Covidien (Medtronic LLC), grants from Alberta Innovates, grants from Heart & Stroke Foundation of Canada, grants from Canadian Institutes of Health Research (Canadian Stroke Prevention Intervention Network), grants from University of Calgary (Hotchkiss Brain Institute), grants from Boehringer Ingelheim Canada, grants from NoNO, Inc., and grants from Biogen. In addition, Dr. Hill has a patent to US Patent office Number: 62/086,077 and licensed and is Stock owner in Pure Web Incorporated and CircleNVI. Dr. Hill is a director of the Canadian Federation of Neurological Sciences, a not-for-profit group, is a director of the Canadian Stroke Consortium, a not-for-profit group, is a director of Circle NeuroVascular, and has received public grant support to the University of Calgary from Alberta Innovates Health Solutions, Canadian Institutes of Health Research, Heart & Stroke Foundation of Canada, and National Institutes of Neurological Disorders and Stroke. The other authors have no conflict of interests.
Statement of Authorship
Dr. Rebchuk contributed to conception, design, data analysis and interpretation, and drafting the manuscript. Dr. Menon provided all oversight and takes full responsibility for the research. Dr. Goyal and Dr. Hill contributed to the conception, design, and data interpretation. All authors critically revised the manuscript and approved the final manuscript.
Supplementary Material
To view supplementary material for this article, please visit https://.doi.org/10.1017/cjn.2021.226.






