Introduction
Status epilepticus (SE) is a serious and potentially life-threatening neurological emergency associated with significant morbidity and mortality. The incidence of SE has been reported to range from 18 to 41 per 100,000 population with an overall mortality rate of about 20%.Reference Betjemann and Lowenstein1 The International League Against Epilepsy defines SE as “a condition resulting either from the failure of the mechanisms responsible for seizure termination or from the initiation of mechanisms which lead to abnormally prolonged seizures (after time point t 1). It is a condition that can have long-term consequences (after time point t 2), including neuronal death, neuronal injury, and alteration of neuronal networks, depending on the type and duration of seizures.”Reference Trinka, Cock and Hesdorffer2 Generally, SE can be defined as >5 min of continuous clinical or electrographic seizure activity or multiple reoccurrences with no return to baseline between episodes. Furthermore, it is well established that a longer seizure duration is associated with worse outcomes.Reference Hocker, Tatum and LaRoche3 Consequently, timely and appropriate management of patients with SE is essential for minimizing morbidity and mortality.
Various antiepileptic drugs (AEDs) can be used in an attempt to terminate seizure activity and control SE, such as benzodiazepines (BDZs), phenytoin (PHT), levetiracetam (LEV), valproic acid (VPA), and phenobarbital (PB). Guidelines have been published to provide clinicians with evidence-based recommendations regarding the treatment of SE. Despite this, there may still be a lack of consistency regarding the choice and dosing of AEDs in the intensive care unit (ICU) as it is indeterminate whether individualizing the choice and dosing of AEDs based on patient-specific factors will help optimize patient outcomes.Reference Muayqil, Rowe and Ahmed4 In addition, aside from initial BDZs, there is a paucity of evidence regarding the efficacy of subsequent AEDs.Reference Brophy, Bell and Claassen5
The objective of this study was to review the critical care management of patients with SE in our ICUs, focusing on the choice, order, and effectiveness of AEDs throughout patients’ ICU stay. In addition, we sought to determine the optimal dosing strategies and predictors of effectiveness of PHT, the most commonly used AED in our institution.
Methods
Design
A retrospective chart review of adult patients with SE admitted to the University of Alberta Hospital, Edmonton, Canada, from January 2015 to December 2016 was conducted. The study was approved by the Health Research Ethics Board of the University of Alberta.
Study Population
Patients’ medical records at the University of Alberta Hospital were requested based on the ICD-10-CA codes for SE. Note that ICD coding in Edmonton still follows the older seizure classification. The codes included were: G41.0 grand mal status epilepticus; G41.1 petit mal status epilepticus; G41.2 complex partial status epilepticus; G41.8 other status epilepticus; and G41.9 status epilepticus, unspecified. Inclusion criteria were adult patients (≥18 years) admitted with SE to the University of Alberta Hospital neurosciences ICU and/or general systems ICU. The exclusion criteria were the following: patients with pseudo-seizures or psychogenic non-epileptic seizures, mortality within 24 h of hospital admission, and patients not admitted to the ICU.
Data Extraction
A thorough review of patients’ records was performed. Data were extracted from paper as well as electronic charts and managed using REDCap database capture tool hosted at the University of Alberta.Reference Harris, Taylor and Thielke6 Information regarding patients’ demographics (age, sex, height, weight), past medical history, admission parameters (presentation, Acute Physiology and Chronic Health Evaluation II [APACHE II], Glasgow Coma Scale [GCS], Sequential Organ Failure Assessment [SOFA] score), alcohol use, admission serum creatinine, and pre-admission AEDs were recorded. Detailed information concerning SE management from admission to ICU discharge, including drugs used, order of AED initiation, AED doses, PHT levels, AED effectiveness, and anesthetic doses and duration, were collected as available. An individual AED was deemed effective if it was the last agent added within 1 day before cessation of seizure activity (clinical and/or electrographic) and weaning off of intravenous (IV) anesthetics. Specific information about patients’ epileptic activity was collected, including history of epilepsy/seizures, documentation of clinical seizures and EEG findings, etiology and type of SE, and duration of SE. SE was defined as >5 min of continuous clinical or electrographic seizure activity or multiple reoccurrences with no return to baseline between episodes. If a patient does not respond to neither initial benzodiazepine nor a following AED, SE was considered as refractory SE. If seizure activity continues or recurs for >1 day after the anesthetic therapy was started, it was considered super-refractory SE (SRSE). Due to the retrospective nature of the study, we were unable to accurately determine the exact duration of SE. Alternatively, we were able to determine whether SE exceeded 2 days in duration. Therefore, the duration of SE was defined dichotomously as ≥2 days based on the documentation of clinical and/or EEG seizures. With respect to etiology, SE was classified as symptomatic when the cause was a known disorder, or cryptogenic when the cause was unknown.Reference Trinka, Cock and Hesdorffer2 Symptomatic SE was further divided into acute, remote, or progressive based on the timeframe between the cause and onset of SE and the nature of etiology.Reference Trinka, Cock and Hesdorffer2 Finally, data regarding each patient’s hospital stay and clinical outcomes were collected, including disposition, ICU and hospital length of stay, and ICU mortality. Data extraction was confirmed by SHM and TL.
PHT levels collected within 24 h of the loading dose (LD) were considered post-LD levels. PHT levels were considered steady-state if the patient was on the same maintenance dose (MD) for ≥3 days. Any other levels were excluded, and clinical judgement among authors was used to interpret all levels. For classification purpose, PHT levels within the range of 40–80 µmol/L were considered therapeutic. Levels <40 and >80 µmol/L were considered sub-therapeutic and supra-therapeutic, respectively. PHT levels were compared using weight-based and standardized dosing. Standardized dosing was defined as PHT LD of 1000 mg and MD of 300 or 400 mg/day.
Data Analysis
Continuous variables were presented as mean ± standard deviation (SD), or median and interquartile range (IQR), where appropriate. Categorical variables were presented as n (%). Continuous variables were compared using Student’s t-test or Wilcoxon rank-sum test, where appropriate. Categorical variables were compared using chi-square or Fisher’s exact test, where appropriate. Adjusted odds ratios (ORs) of predictors of PHT effectiveness were determined using multivariate logistic regression analysis. The fit of the final model was confirmed using Hosmer–Lemeshow (HL) goodness-of-fit test, and model discrimination was confirmed using the area under the receiver operating characteristic (ROC) curve. Missing data, if any, were handled by complete case analysis. Level of significance was set at p < 0.05. Statistical analysis was done using STATA, version 15 (STATA Corp, College Station, TX). Figures were plotted using GraphPad Prism, version 7 (GraphPad Software, La Jolla, CA).
Results
Study Participants
Medical records of a total of 103 patients were identified, 58 of whom were admitted to the ICU. Two records were not available, and one patient was admitted twice during the study period and therefore contributed as two separate admissions. Accordingly, 56 records were included in the present study.
Baseline Characteristics
Patients’ baseline characteristics are presented in Table 1. Mean age was 56 years with 22 females (39%). Mean BMI was 27 kg/m2. Almost two-thirds (63%) of our study population had a history of seizure and/or epilepsy with 26 (46%) patients taking pre-admission AEDs. The most common pre-admission AED was LEV (n = 10), followed by PHT (n = 7), and carbamazepine (n = 7). Pre-admission AEDs were continued upon admission in 20 patients, and their dose were increased in 7 patients. Those AEDs were deemed effective, ineffective, and of unclear efficacy (due to concomitant initiation of other agents) in 8, 6, and 6 patients, respectively. The most common SE type and possible etiology were tonic-clonic SE (n = 36) and cerebrovascular (n = 18), respectively. Furthermore, the most common etiology classification was acute symptomatic (57%), followed by remote symptomatic (27%), cryptogenic (11%), and progressive symptomatic (5%).
Table 1: Baseline characteristics of patients

SCr = serum creatinine; COPD = chronic obstructive pulmonary disease; MI = myocardial infarction; APACHE II = Acute Physiology and Chronic Health Evaluation; GCS = Glasgow Coma Scale; SOFA = Sequential Organ Failure Assessment; AED = antiepileptic drug; NCSE = non-convulsive status epilepticus; NORSE = new-onset refractory status epilepticus; RSE = refractory status epilepticus; SRSE = super-refractory status epilepticus.
a n = 55.
b Patients may have taken more than one pre-admission AED.
c Patients may have more than one type of SE.
d Patients may have more than one possible SE etiology.
Choice, Order, and Effectiveness of Newly Started AEDs
BDZs were initially given in 89% of our patients. Following BDZs, PHT was the most commonly initiated agent, being administered in 44 patients (79%), followed by LEV (n = 11, 20%) (Table 2). In addition, PHT and LEV were the most frequently used first- and second-line agents, respectively (Figure 1). PHT was used primarily in tonic-clonic seizures (n = 30) with a median LD of 1000 mg followed by an initial median MD of 400 mg (IQR 100), and it was deemed effective in 30 patients (68%). LEV MD ranged from 1000 to 3000 mg/day, and it was deemed effective in five patients (45%). LEV was also the most common switch therapy after seizure termination was achieved, which occurred in six patients.
Table 2: Summary of choice, order, and effectiveness of patients newly started on antiepileptic drugs in SE
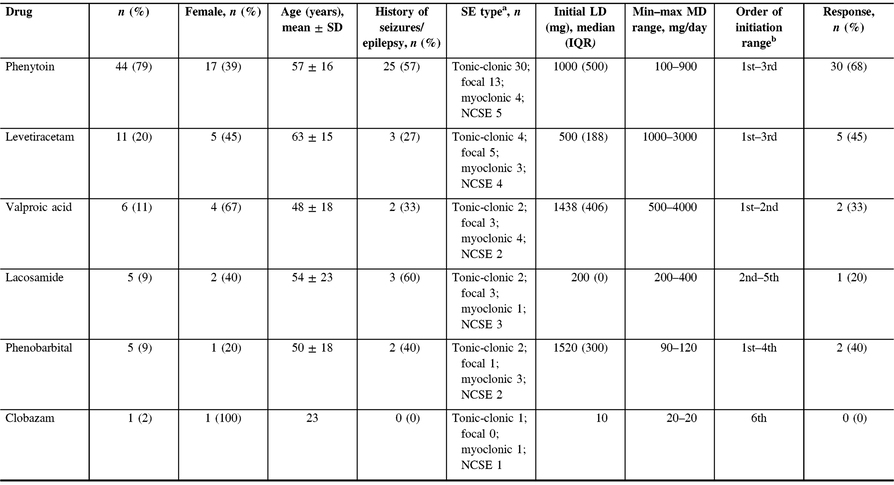
SE = status epilepticus; NCSE = non-convulsive status epilepticus; LD = loading dose; MD = maintenance dose.
a Patients may have more than one SE type.
b The order of AED initiation was indeterminate in two patients and therefore they were not included.
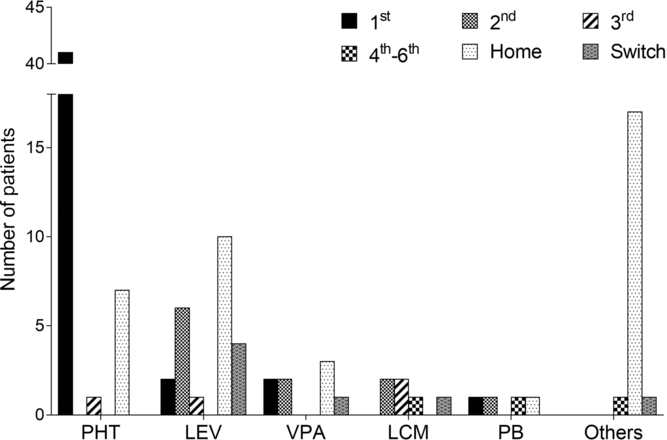
Figure 1: Sequence of administered antiepileptic drugs (AEDs) following benzodiazepines, from hospital admission to intensive care unit discharge. An agent reported as a pre-admission AED and that was continued during the hospital stay was referred to as home. An agent that was started after successful seizure termination for step-down therapy or for intolerable adverse effects was referred to as “switch.” Others include carbamazepine, clobazam, perampanel, topiramate, and lamotrigine. The order of AED initiation was indeterminate in two patients and therefore they were not included. PHT, phenytoin; LEV, levetiracetam; VPA, valproic acid; LCM, lacosamide; PB, phenobarbital.
Hospital Course and Clinical Outcomes
As shown in Table 3, almost 43% of our patients were discharged without putting them on support services. In addition, 13 (23%) patients died: SRSE (n = 3), anoxic brain injury/SRSE (n = 2), anoxic brain injury (n = 1), sepsis (n = 1), bacterial meningitis (n = 1), hepatic encephalopathy (n = 1), and neurological deterioration of unclear cause (n = 4). The mean ICU length of stay was 6.5 days, with anesthetics being initiated in 48 patients (86%) for a mean duration of 2.9 days. SE was sustained for ≥2 days in over one-third (38%) of our patients.
Table 3: Hospital course and clinical outcomes

ICU = intensive care unit.
a Including home medications.
PHT Effectiveness
Since PHT was the most commonly used agent, we looked into determining the predictors of its effectiveness. Therefore, we compared the baseline characteristics of patients who achieved seizure remission while on PHT (i.e., effective) with those who did not (Table 4). Patients in which PHT was effective had a lower BMI compared with those in which it was not effective (25 ± 6 vs. 30 ± 10, respectively, p = 0.05). Additionally, patients who had a previous history of seizures and/or epilepsy were more likely to achieve seizure termination while on PHT compared with those with no previous history (67% vs. 36%, p = 0.05). Likewise, in patients with tonic-clonic seizures, PHT was more likely to be effective (50% vs. 77%, p = 0.08).
Table 4: Comparison between a group of patients in which phenytoin was effective and another in which phenytoin was not effective

BMI = body mass index; LOS = length of stay; APACHE = Acute Physiology and Chronic Health Evaluation; GCS = Glasgow Coma Scale; SOFA = Sequential Organ Failure Assessment; SE = status epilepticus; LD = loading dose; ICU = intensive care unit; AED = anti-epileptic drug.
a n = 29.
b We are missing data regarding the initial phenytoin loading dose in five patients.
c Including home medications; significance level set at p <0.05.
Table 5 depicts the crude and adjusted ORs for predictors of PHT effectiveness in the best-fit logistic regression model in patients newly started on PHT (ROC AUC 0.765, HL test not significant). As shown in the table, tonic-clonic SE was an independent predictor of PHT effectiveness (OR 5.01, 95% CI 1.02–24.7, p = 0.048). In addition, obese individuals tend not to respond to PHT, but this did not reach statistical significance in the final model (OR 0.16, 95% CI 0.03–1.07, p = 0.059). However, obesity was statistically significant in the model adjusted for tonic-clonic SE only (OR 0.15, 95% CI 0.03–0.85, p = 0.032, ROC AUC 0.721, HL test not significant).
Table 5: Adjusted odds ratios for predictors of phenytoin effectiveness

BMI = body mass index; OR = odds ratio; CI = confidence interval; SE = status epilepticus.
Significance level set at p < 0.05.
PHT Dosing and Levels
PHT weight-based load dosing ≥20 mg/kg occurred in only seven patients (18%); however, 22 patients (56%) received PHT LDs ≥15 mg/kg. A total of 126 PHT levels were identified from 38 patients. After review, we included 45 levels from 28 patients: 19 were considered post-LD levels and 26 were considered at steady state (Table 6). As shown in Figure 2, more patients reached PHT levels within the reference range (40–80 µmol/L) when given a weight-based LD ≥15 mg/kg compared with a standard 1000 mg LD (60% vs. 29%, respectively). This suggests that loading PHT based on weight is better compared with standard doses; however, this difference did not reach statistical significance. Conversely, a standard MD of 400 mg/day and a weight-based MD >5 mg/kg per day both resulted in 70% of patients achieving levels within the reference range. However, when a standard MD of 300 mg/day was used, patients reaching PHT levels between 40 and 80 µmol/L dropped to 25%. Of note, in three patients, administration routes were switched from IV to oral on the same MD; they experienced a statistically significant reduction in steady-state levels from 71 ± 10 to 54 ± 6 µmol/L (p = 0.025) within 2–4 days.
Table 6: Comparison of phenytoin levels after weight-based and standard intravenous dosing

PHT = phenytoin; LD = loading dose; MD = maintenance dose.
a Number of phenytoin levels.
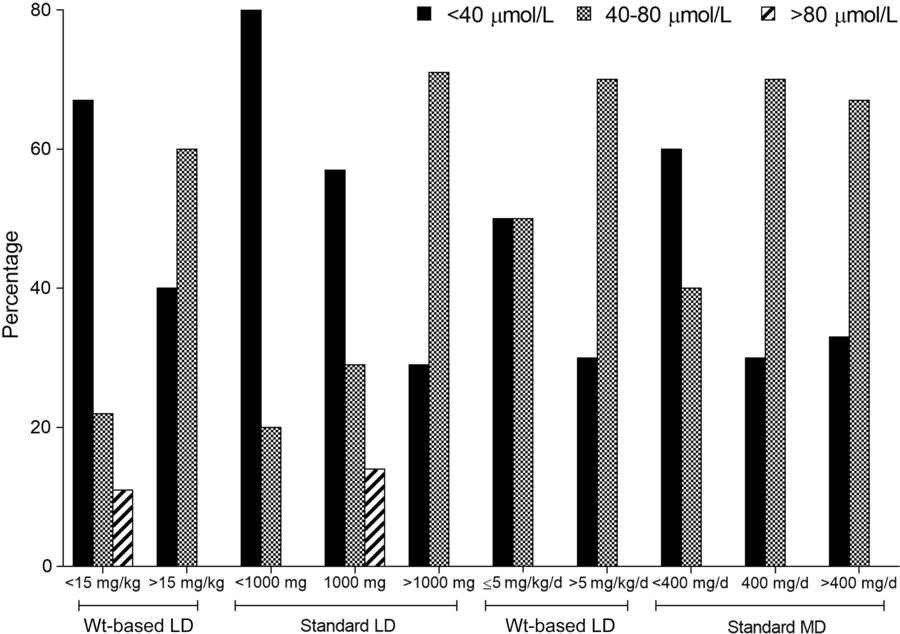
Figure 2: Percentage of patients reaching different phenytoin level categories based on intravenous loading dose (LD) and maintenance dose (MD) being weight-based or standardized.
Discussion
SE is a neurological emergency associated with devastating consequences and must be promptly treated. Despite the severity of the condition, there is a lack of data supporting the preferred choice, order, and effectiveness of AEDs as well as the effect of individualizing treatments based on patient-specific factors. The results of the present study suggest that the use of PHT, the most commonly used AED in our institution, can be optimized and potentially individualized on a patient-to-patient basis.
Due to methodological differences, inherent difficulty in defining and diagnosing SE, as well as its low incidence, the reported etiology and outcomes may vary widely among studies. Our patients had a mean age of 56 years, almost two-thirds (63%) of them having a previous history of epilepsy/seizures. Both are consistent with what has been previously reported in literature.Reference Towne, Pellock and Ko7–Reference Legriel, Mourvillier and Bele9 In addition, we found that cerebrovascular origin, low AED levels, and non-compliance were the most common possible etiologies, similar to previous studies.Reference Betjemann and Lowenstein1,Reference Legriel, Mourvillier and Bele9 While the specific causes of SE may vary among studies, it is generally accepted that acute symptomatic causes of SE are the most common,Reference Betjemann and Lowenstein1 which mimics our study. Moreover, mortality in the present study was similar to what has been previously reported (∼20%).Reference Betjemann and Lowenstein1,Reference Towne, Pellock and Ko7,Reference Legriel, Mourvillier and Bele9,Reference Lowenstein and Alldredge10
BDZs were initially given in 89% of our patients; however, due to the retrospective nature of our study, data may be missing. This is in parallel with the guidelines as extensive evidence has supported the initial use of BDZs.Reference Brophy, Bell and Claassen5,Reference Glauser, Shinnar and Gloss11 Additionally, the guidelines have recommended PHT, LEV, VPA, and PB as urgent control therapy following BDZs.Reference Brophy, Bell and Claassen5,Reference Glauser, Shinnar and Gloss11 While there is presently insufficient evidence on the efficacy of one agent over another, it has been suggested that PHT may be preferred for most patients.Reference Brophy, Bell and Claassen5 Similarly, in line with the guidelines, PHT is the most commonly initiated first-line agent and was found to be effective at terminating seizures in 68% of patients newly started on AED in our institution. This result is consistent with what has been previously reported in literature (59–68%).Reference Alvarez, Januel and Burnand12,Reference Chakravarthi, Goyal and Modi13 Furthermore, a meta-analysis of 22 studies looking at the effectiveness of multiple AEDs in SE has reported the efficacy of PHT at 50.2%.Reference Yasiry and Shorvon14
We identified three variables that are possibly associated with PHT’s effectiveness. To our knowledge, there are currently no studies evaluating the predictors of PHT effectiveness in SE. First, tonic-clonic SE was shown to be an independent predictor of PHT effectiveness in multivariate regression (OR 5.01, 95% CI 1.02–24.7, p = 0.048). Generalized convulsive SE (GCSE), which includes tonic-clonic SE, is the most common and life-threatening type of SE; however, it is typically clearly identified and thus can be promptly treated.Reference Gaitanis and Drislane15 In general, non-convulsive SE (NCSE) has better outcomes compared with GCSE; however, it is hard to recognize, usually requiring an EEG, and therefore treatment delays are possible.Reference Gaitanis and Drislane15 The Veterans Affairs Cooperative Study has reported a median duration of SE at enrollment at 2.8 h for GCSE and 5.8 h for subtle SE.Reference Treiman, Meyers and Walton16 In addition, it is well known that a longer seizure duration is associated with worse outcomes and treatment failure.Reference Betjemann and Lowenstein1,Reference Hocker, Tatum and LaRoche3 With this is mind, it is uncertain whether tonic-clonic SE is predictive of PHT effectiveness or successful seizure termination in general; consequently, more studies are needed to inform this finding.
Second, a previous history of epilepsy/seizures was shown to be associated with PHT effectiveness. It has been reported that a history of seizures/epilepsy or etiology of low AED levels are determinants for lower mortality in SE.Reference Towne, Pellock and Ko7,Reference Gaitanis and Drislane15,Reference Rossetti, Hurwitz and Logroscino17 Building on this finding in a prospective study, authors attempted to create a Status Epilepticus Severity Score (STESS) tool and indicated that a history of previous seizures is a protective independent variable associated with mortality (OR 0.23, CI 0.08–0.65, p = 0.006).Reference Rossetti, Logroscino and Milligan18 We hypothesize that patients with a history of seizures/epilepsy may be treated more promptly and aggressively due to faster diagnosis, which may positively impact outcomes. It is possible that a history of seizures/epilepsy could be a confounding variable by indication. Similar to the finding above, it is difficult to determine whether PHT is more effective in these individuals, or if a history of seizures/epilepsy is advantageous for overall treatment effectiveness, and therefore more investigation is required to explore this association.
Third, obese patients (BMI > 30 kg/m2) were less likely to achieve seizure remission while on PHT. From our study, it is uncertain why obese individuals responded poorly to PHT. We did not have sufficient sample size to determine whether the reduced response in obese individuals is independent of PHT levels. However, we postulate that it may be due to an interplay of multiple factors. The common practice of standard LDs may be leading to underdosing, and thus weight-based LDs are strongly recommended. In addition, pharmacokinetic and pharmacodynamic properties of PHT as well as potential unknown factors may be influencing its effectiveness in this population. To our knowledge, currently there is no published study specifically looking at the use of PHT in treating obese individuals with SE. Over 30 years ago, Abernethy et al.Reference Abernethy and Greenblatt19 explored PHT disposition in obesity and determination of an appropriate LD. The authors reported an increased volume of distribution (V d) in obese individuals. Therefore, they derived that LDs should be based on ideal body weight (IBW) with a special dosing consideration for obese individuals. They proposed that 1.33 times any weight exceeding IBW should be added to the typical LD.Reference Abernethy and Greenblatt19 More recently, a retrospective study was conducted regarding the influence of obesity and sex on fosphenytoin/PHT LDs. The authors concluded that fosphenytoin/PHT LDs should be at least 15 mg/kg of actual body weight, and obese women specifically require at least 20 mg/kg.Reference DasGupta, Alaniz and Burghardt20 They hypothesized that a higher body fat in women than in men, and due to the lipophilicity of PHT, leads to an increased V d requiring higher doses. In addition, Clark et al. further explored fosphenytoin in obese individuals and concluded that the current guidelines of administering weight-based LDs without adjustment for patient obesity did not affect clinical outcomes.Reference Clark, Leloux and Dierkhising21 It is important to note that patients in this study had a median LD of 19 mg/kg, likely influencing treatment success.Reference Clark, Leloux and Dierkhising21 The severity of SE in conjunction with the high rates of obesity represents a large population that may be vulnerable to treatment failure, and thus, more studies are urgently required to explore this association.
Lastly, we explored weight-based and standardized dosing of PHT to determine the optimal dosing strategy. A weight-based LD of 20 mg/kg is currently recommended in the guidelinesReference Brophy, Bell and Claassen5; however, only seven of our patients (18%) received PHT doses reaching this target. The high percentage of patients who received an inadequate LD could be explained by the use of 1000 mg standard LD in one-third of our patients. When these doses were examined in conjunction with available PHT levels, patients receiving a weight-based LD >15 mg/kg were more likely to have PHT levels within the reference range (Figure 2) compared with patients receiving a standard LD of 1000 mg; however, this did not reach statistical significance. However, this finding has been previously supported and the authors have recommended avoidance of preset PHT LDs.Reference Selioutski, Grzesik and Vasilyeva22 This trend is further explained in a study by Brancaccio et al.,Reference Brancaccio, Giuliano and McNorton23 which suggested that a fixed dosing strategy of 1000 mg may be used because of prescriber familiarity; however, with the rates of obesity increasing, this approach may lead to a large proportion of patients failing to achieve therapeutic concentrations, subsequently impacting patient outcomes. Interestingly, Brancaccio et al. explored the impact of a PHT LD program, which was further stratified into pharmacist-led and prescriber-led. They showed that pharmacist-led dosing programs significantly improved the proportion of patients who received optimal PHT LDs in comparison to prescriber-led programs (82% vs. 49%), and that a 1000 mg LD was used less frequently in the pharmacist-led group (42.9 vs. 65.1%).Reference Brancaccio, Giuliano and McNorton23
A similar trend was found for PHT MDs; however, it was not as clear. Patients who received a standard dose of 400 mg/day or a weight-based dose >5 mg/kg per day showed a trend to be equally likely within therapeutic range. On the contrary, patients who received a standard dose of 300 mg/day were less likely to be at target. The use of an initial PHT dose of 300 mg/day has been recommended in drug monographs and other drug guides.24–26 While it may be appropriate to initiate a patient with epilepsy on 300 mg/day, critically ill patients with SE may need higher doses, as seen in our study. It is important to mention that PHT levels measured ≥3 days on a stable dose were considered steady-state levels. With IV LDs given to patients, steady state was expected to be attained sooner than 3 days. However, if MD is suboptimal, it may take 3–5 half-lives for PHT to reach a steady state. PHT is a drug with dose-dependent pharmacokinetics, with an average half-life ∼20–24 h, or longer depending on the drug dose and drug-specific kinetics in individual patients. Therefore, on average, it may take 3–5 days for PHT to reach steady state and ideally in 7 days. Therefore, some of the measured levels may not reflect a steady state. However, in the setting of SE and critical illness, waiting for a week or longer might be too late. As a result, pre-steady-state levels were used to gauge how sufficient is the MD and to top up the dose as needed. Furthermore, it has been suggested that when PHT is used in SE, oral therapy should replace IV as soon as possible.24,25 IV PHT was switched to the oral form at the same MD in three patients in the ICU. These patients had statistically significant reductions in their PHT levels, ranging from 20% to 29% (p = 0.025) within 2–4 days. Although these levels might not reflect a steady state, the downward trend in PHT levels suggests the possibility of reduced exposure secondary to altered PHT absorption and clearance. PHT is characterized by variable absorption, high protein binding, saturable metabolism, and multiple interactions contributing to its complex pharmacokinetic profile.Reference Richens27 With all of this in mind, it may be reasonable to conclude that minor changes in PHT dosing can have drastic implications on PHT levels and potential outcomes. This is in addition to the potential interaction of PHT with tube feeding. Therefore, it is recommended to hold feeding 2 h before and after PHT administration.Reference Bauer28 While it is still inconclusive whether these reductions in PHT levels have an impact on patient outcomes, it may be reasonable to recommend that patients in the ICU should not be switched to oral medications due to their critical condition. In addition, in patients whose PHT is switched from IV to oral dosing, monitoring following the switch is essential.
Due to the retrospective nature of the study, an association between drug levels and seizure remission was difficult to be determined. Some patients might respond to PHT at concentrations well below the recommended range, whereas others might require higher levels to achieve seizure control.Reference Patsalos, Berry and Bourgeois29 This emphasizes the importance of “treating the patient, not the level,” as it is imperative to take both seizure activity and PHT levels into consideration. While it has become common practice to use drug monitoring to individualize PHT doses, the impact on patient outcomes has seldom been addressed.Reference Patsalos, Berry and Bourgeois29 Our results emphasize the need for prospective studies with more accurate documentation of seizure activity in relation to PHT doses and levels to describe this association.
The main limitation of the present study was the retrospective design. A retrospective study implies a risk of missing or incorrectly extracted data. Moreover, the fact that a large amount of data were abstracted from handwritten charts further exacerbates this risk. Nevertheless, we used a standardized form for data collection to limit inconsistency. Owing to the small sample size and the low frequency of use of AEDs other than PHT, it was difficult to assess their effectiveness in the setting of SE. Moreover, since our inclusion criteria included only those who were admitted to the ICU, most likely those patients were administered at least an additional AED following the initial BDZ. As result, we were unable to assess the efficacy of the initial BDZ based on our working definition of efficacy. Similarly, we used anesthetics weaning to gauge the efficacy of other AEDs. As a result, we were unable to assess their efficacy separately from other AEDs. However, 8 out of 47 patients (17 %) on anesthetics progressed into SRSE, which generally indicates failure of anesthetics and concomitant AEDs in those patients. In addition, the retrospective design made it difficult to accurately determine SE duration and associations between AED doses and outcomes. However, this study provides a good snapshot of the critical care management of SE in a teaching hospital and may help to influence the direction of future studies. It enforces the need for prospective studies to further explore the use of AEDs following BDZs, including the newer agents, as well as to investigate the predictors of PHT effectiveness and its optimal dosing strategies.
Conclusion
Overall, the critical care management of SE in our institution was consistent with the guidelines. Our findings stress that standardized PHT LDs should be avoided and the agent should be loaded according to the guidelines at 20 mg/kg to achieve levels within the reference range. Moreover, standard MDs of 300 mg/day should likely be avoided and doses of at least 400 mg/day or ≥5 mg/kg per day should be used. To help support these findings, more studies are needed to define the association between PHT dosing, PHT levels, and patient outcomes in the setting of SE. Finally, investigations into the predictors of PHT effectiveness, specifically its use in obese individuals, are necessary to help individualize patient care and optimize patient outcomes.
Disclosures
The authors have no conflicts of interest to declare.
Statement of Authorship
SHM and NA contributed to study design. SHM and TL conducted data collection. SHM, VM, and TL conducted data clean-up and analysis. All authors interpreted data analysis results and wrote the manuscript.










