Neuroendoscopy is an important minimally invasive technique in neurosurgeryReference Grunert, Gaab and Hellwig 1 and has evolved to include a role in both the diagnosis and treatment of a variety of disorders.Reference Cappabianca, Cinalli and Gangemi 2 In a historical context, the first therapeutic neuroendoscopic case was reported in Chicago in 1910, when L'Espinasse used a cystoscope to fulgurate the choroid plexus of two infants with hydrocephalus.Reference Abbott 3 Choroid plexus cauterization for the treatment of hydrocephalus gained popularity in the 1930s,Reference Harris 4 followed by endoscopic third ventriculoscopy (ETV) in the 1960s.Reference Enchev and Oi 5 However, it was not until the 1990s that endoscopy began to establish itself as a routine neurosurgical procedure.Reference Di Rocco, Cinalli, Massimi, Spennato, Cianciulli and Tamburrini 6 It grew from purely intraventricular applications to include surgery within other fluid-filled cavities such as the cisterna magna and the spinal canal, and eventually outside the cranial space to include skull base surgery,Reference Chrastina, Novak and Riha 7 tumor biopsy and resection, exploration of the cerebellopontine angle, and treatment of craniosynostoses.Reference Van Rompaey, Bush, McKinnon and Solares 8 , Reference Jenkins, Smith and McNeely 9 Neuroendoscopy may be done via existing anatomical pathways (e.g. transnasal) or using transcortical single- or multiport systems, in addition to robotic applications.Reference Vogel, Manjila and Cohen 10 , Reference Zada and Liu 11
As experience grows and techniques are refined, complication rates are decreasing and more and more neurosurgeons are enticed by the less-invasive appeal of the neuroendoscope. However, despite these advances and novel initiatives to train growing numbers of surgeons in the proper use of this technique,Reference Agrawal, Kato, Sano and Kanno 12 , Reference Filho, Coelho, Cavalheiro, Lyra and Zymberg 13 intracranial neuroendoscopy is still seen as a pediatric neurosurgery procedure, even though there are numerous indications in adult patients. We report a large series of neuroendoscopic cases in which the same surgeons performed both adult and pediatric procedures to illustrate the similarities and differences between pediatric and adult patient populations.
Methods
Following institutional board approval (E-22666), a retrospective chart review was performed for all patients who underwent neuroendoscopic surgery at the Foothills Medical Centre and the Alberta Children’s Hospital between 1994 and 2008, as identified from a patient-specific database. These two hospitals are tertiary care centers providing care to all neurosurgical patients in Southern Alberta and parts of South-Eastern British Columbia (population approximately 1.5 million). We excluded transnasal pituitary and skull base procedures.
Information was collected from operative reports, clinic notes, progress notes in hospital, and discharge summaries. Recorded data included patient demographics, presenting symptoms, procedures performed, complications, and follow-up times. Pediatric patients were defined as age ≤17 years; adult patients were defined as age ≥18 years. Major complications included death, permanent neurological deficit or endocrine abnormality, meningitis, and a subdural collection requiring shunting. Minor complications included cerebrospinal fluid (CSF) leak, seizure, wound infection, and transient neurological deficit or endocrine abnormality.
Statistical analysis was performed using the R programming environment (Vienna, Austria). A p value of less than 0.05 was predefined to indicate a statistically significant result.
Results
A total of 330 procedures were performed in 273 consecutive patients between 1994 and 2008; these comprised 141 procedures in 112 pediatric patients and 189 procedures in 161 adult patients. Both adult and pediatric cases were performed by the same surgeons. ETV was the most common procedure performed in both groups. As expected from disease presentations and characteristics, more colloid cysts were resected in adults and more arachnoid cysts were fenestrated in children. Mean follow-up duration after the neuroendoscopic procedure was 12.9 years (range 5.7-19.7 years excluding one early death) and median follow-up 12.8 years.
A breakdown of the adult procedures and their complications is shown in Table 1. As seen in the table, ETV was the most common procedure performed in adult patients, followed by colloid cyst resection, tumor biopsy, and cyst fenestration. Major complications occurred in eight of 189 procedures, giving a major complication rate of 4.23%. These included four cases of meningitis and three cases of permanent neurological deficit, which included one postoperative case of hemiparesis, one cranial nerve palsy, and one patient who developed chronic seizures. In addition, one death occurred following septostomy in a 55-year-old man with a ventriculoperitoneal shunt. His shunt failed because of ventriculitis and a cerebellar abscess, and following the urgent removal of the shunt, a septostomy, and placement of an extraventricular drain, he developed a large intracerebral hemorrhage around the drain. Given his overall poor state, care was withdrawn.
Table 1 Adult endoscopic procedures and their major complications
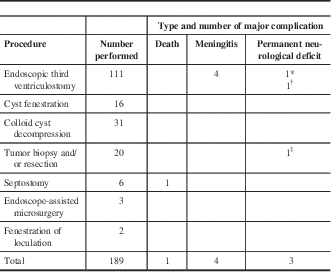
* Cranial nerve palsy.
† Chronic seizures.
‡ Hemiparesis.
A breakdown of the pediatric procedures and their complications is shown in Table 2. As seen in the table, ETV was the most common procedure performed in pediatric patients, followed by cyst fenestration. Major complications occurred in eight of 141 procedures, giving a major complication rate of 5.67%. These included two cases of meningitis, two patients who developed permanent and severe weight gain, one patient with panhypopituitarism, one patient with severe memory impairment, and two patients who developed subdural collections that required shunting.
Table 2 Pediatric endoscopic procedures and their major complications
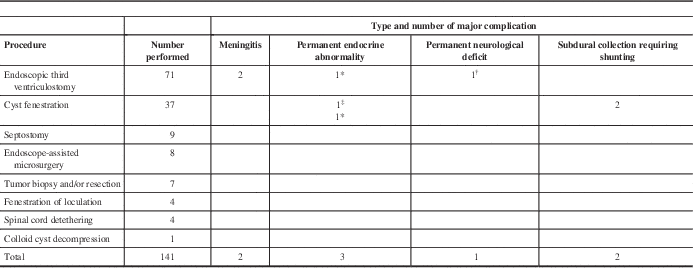
* Obesity.
† Memory deficit.
‡ Panhypopituitarism.
A breakdown of all minor complications in both pediatric and adult patients is shown in Table 3. Minor complications occurred in 27 of 330 total procedures, giving an overall minor complication rate of 8.18%. The most common of these were CSF leak and transient seizures, followed by wound infection, transient weight gain or electrolyte abnormality, and transient cranial nerve III palsy.
Table 3 Minor complications of all procedures

* Cranial nerve III palsy.
† Hemiparesis.
‡ Includes four spine detetherings and six fenestrations of loculation.
We have previously reported the success of ETV to treat hydrocephalus as defined by remaining shunt-free at 1 year postprocedureReference Hader, Walker, Myles and Hamilton 14 with rates of 82.5% for primary ETV and 82% for ETV done in the setting of shunt failure and subsequent shunt removal. The success rate for this expanded population is essentially unchanged (81%), and only three delayed failures of ETV have occurred with long-term follow-up. All three delayed failures occurred in patients who underwent their ETV when younger than age 18. Two occurred within 2 years and one 10 years post-ETV as an adult. All underwent successful repeat ETV procedures and remain shunt-free.
To determine if there was a difference in the incidence of complications between adult and pediatric patients undergoing specific neuroendoscopic procedures, two-tailed chi-square tests were applied to major complication rates for each type of procedure, but none reached statistical significance (Table 4), nor did the overall complication rate between adult and pediatric patients (4.2% vs 5.7%, p=0.547).
Table 4 Differences in major complication rates by procedure
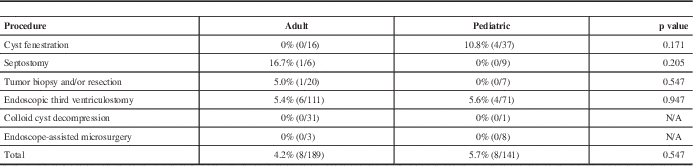
Incidence of major complication rates (number of major complications per total number of procedures) in endoscopic procedures for adult versus pediatric patients. The p values calculated using two-tailed chi-square test (alpha=0.05).
Univariate logistic regression analysis was used to attempt to identify any specific procedures that may potentially be associated with major complications, and to determine if there was a relation between patient age and complication rates, but neither reached statistical significance (odds ratios, 0.62-1.43; p values, 0.22-0.79).
Discussion
As minimally invasive approaches grow in popularity, endoscopy is becoming a powerful tool in the neurosurgeon’s armamentarium.Reference Hardavella and Ianovici 15 However, despite the appeal of a smaller incision, and the now well-described anatomy of intraventricular spaces,Reference Longatti, Fiorindi, Feletti, D'Avella and Martinuzzi 16 , Reference Longatti, Fiorindi, Perin and Martinuzzi 17 serious perioperative complications may occur. This is largely due to the proximity of the endoscope to critical neural structures, as is the case in several endoscopic procedures.Reference Cinalli, Spennato and Ruggiero 18 , Reference Ganjoo, Sethi, Tandon, Singh and Pandey 19 However, the incidence of complications is low, as reported in our population-based study, and varies with operative indication as well as patient age. Despite our finding that there is no statistically significant difference in complication rates between adult and pediatric patients, neuroendoscopy maintains the reputation of being a largely pediatric technique.
This large study is the first, to our knowledge, to report on endoscopic cases wherein the same surgeons performed both the pediatric and adult procedures, allowing for a direct and meaningful comparison of complication rates between the two patient groups. It is also the first to provide significant long-term follow-up.
Here we discuss the complications and outcomes of neuroendoscopy through its currently established indications.
Hydrocephalus
Hydrocephalus is a heterogeneous group of disorders that spans a wide range of ages and symptom profiles.Reference Bergsneider, Miller, Vespa and Hu 20 As such, each patient requires an individualized diagnostic and treatment approach. Traditionally, the treatment of hydrocephalus involved ventricular shunting, but because of the tendency of those systems to fail over time, whether from shunt malfunction or infection,Reference Jallo, Kothbauer and Abbott 21 endoscopic techniques are emerging as possible alternatives. These include ETV and septostomy, options that are most suitable for treating those patients with obstructive hydrocephalus.Reference Bergsneider, Miller, Vespa and Hu 20 - Reference Vogel, Bahuleyan, Robinson and Cohen 30 ETV consists of creating a hole through the tuber cinereum in the floor of the third ventricle to allow CSF to flow into the interpeduncular and prepontine cisterns, thereby bypassing obstructions in the aqueductal area,Reference Jallo, Kothbauer and Abbott 21 , Reference Hellwig, Grotenhuis and Tirakotai 31 - Reference Brockmeyer 40 whereas septostomy involves placing the hole in the septum pellucidum. Another postulated mechanism involves the restoration of the pulsatility of ventricular walls as a result of the stoma, an effect that may help restore CSF dynamics by driving CSF flow from the ventricular system into the subarachnoid space.Reference Gangemi, Maiuri, Colella, Magro, Seneca and de Divitiis 22 On occasion, both ETV and septostomy may be required for the same patient to treat hydrocephalus.
The overall success rate of ETV has been reported as high as 89%,Reference Kadrian, van Gelder and Florida 23 and possibly even higher in the subset of patients with aqueductal stenosis,Reference Costa Val, Scaldaferri and Furtado 41 with most failures presenting in the first few weeks after surgery.Reference Santamarta, Diaz Alvarez and Goncalves 24 The long-term success for ETV in this pediatric and adult patient population was 81%. Various classification systems are emerging not only to help predict outcome after endoscopic procedures for hydrocephalus, but also to aid in the selection of appropriate patients to maximize positive outcomes.Reference Kulkarni, Drake and Mallucci 25 , Reference Kulkarni, Drake and Kestle 42 - Reference Ros, Romero and Ibanez 45
The most common indication for ETV is aqueductal stenosis, either from congenital narrowing of the aqueduct or obstructive tumors such as tectal gliomasReference Javadpour and Mallucci 46 and obstructive cysts.Reference Farin, Aryan, Ozgur, Parsa and Levy 47 , 48 Other indications include the fenestration of loculated compartments, 49 , Reference Oi and Abbott 50 ventriculoperitoneal shunt malfunction,Reference Bilginer, Oguz and Akalan 26 , Reference O’Brien, Javadpour, Collins, Spennato and Mallucci 27 and preresectional ETV for posterior fossa tumors.Reference Bhatia, Tahir and Chandler 51 Yet another potential role for ETV is in the realm of communicating hydrocephalusReference Hailong, Guangfu and Haibin 52 —specifically normal pressure hydrocephalusReference Gangemi, Maiuri, Buonamassa and Colella 53 , Reference Paidakakos, Borgarello and Naddeo 54 —although these remain controversial indications. A recent randomized, open-label trial of 42 patients with normal pressure hydrocephalus found that those treated with a ventriculoperitoneal shunt had significantly better neurological outcomes at 12 months compared with those who underwent ETV (77% vs 50%),Reference Pinto, Saad and MFd 55 whereas a large population-based study using institutional data found that ETV is associated with greater perioperative mortality and complication rates in these patients compared with shunting.Reference Chan, McGovern and Zacharia 56 Although more needs to be learned about this patient population, and the benefits of ETV seem inferior to shunting in hydrocephalic people with normal or low pressures, it may still play a role in appropriately selected patients.Reference Hamilton and Price 57
Appropriate candidates for ETV include both adults and children, although infants younger than 1 year old tend to have worse outcomes,Reference Kadrian, van Gelder and Florida 23 , Reference Tisell, Almstrom, Stephensen, Tullberg and Wikkelso 58 - Reference Drake, Kulkarni and Kestle 62 especially if they are born preterm or with a low birth weight.Reference Elgamal, El-Dawlatly, Murshid, El-Watidy and Jamjoom 63 Our series only had 34 children younger than 1 year of age undergoing a neuroendoscopic procedure, and only 14 underwent ETV. Complication rates in our pediatric population differed slightly from the literature in that, whereas overall trends showed complication rates increased with decreasing age, the highest rates of complication occurred in the 5- to 10-year-old age range.
In our series, 182 third ventriculostomies and 15 septostomies were performed; of these 197 procedures, 80 were performed on pediatric patients and 117 on adult patients. The overall major complication rate was 5.6%, which is similar to that of other studies. A recent systematic review by Bouras and Sgouros examined 2985 ETV procedures and reported an overall complication rate of 8.5%, a permanent morbidity rate of 2.4%, and a mortality rate of 0.21%.Reference Bouras and Sgouros 64 Jenkinson reported a 5.8% complication rate in 190 adult ETV proceduresReference Jenkinson, Hayhurst, Al-Jumaily, Kandasamy, Clark and Mallucci 65 and Kadiran described a 4.9% infection rate, 7.2% transient major complication rate, and a 1.1% permanent complication rate in 203 ETV patients.Reference Kadrian, van Gelder and Florida 23 In general, it has been reported that complication and mortality rates decrease with increased surgeon experience and are lower compared with open procedures in experienced hands.Reference Farin, Aryan, Ozgur, Parsa and Levy 47 , Reference Egger, Balmer, Altermatt and Meuli 66 , Reference Brockmeyer, Abtin, Carey and Walker 67 A qualitative analysis of our study did show that complication rates marginally decreased as surgeon experience increased, but it is important to note that equipment quality also improved over the course of the series. There were also more adult cases done later in the series, which may have contributed to the lower, albeit nonsignificant, complication rate in adult versus pediatric patients (4.2% vs 5.7%).
In addition, complications have been reported to occur more frequently in previously shunted patients than in patients treated for newly diagnosed hydrocephalusReference Hader, Walker, Myles and Hamilton 14 as well as patients undergoing repeat procedures.Reference Hader, Walker, Myles and Hamilton 14 , Reference Ersahin and Arslan 68 However, many of these complications are classified as minor, are transient, and may not affect long-term outcome. Therefore, when assessing the risks versus benefits of performing ETV in previously shunted patients, it is important to take into consideration the natural history of these patients when using a shunt, and subsequently their cumulative risk of shunt revision and infection over the lifespan of the shunt.
Overall, the complication rate for ETV is low and is similar to the expected infection rate associated with shunts.Reference Hader, Walker, Myles and Hamilton 14 Although the potential exposure to significant neurological complications with ETV may be higher than the risk of a single shunt operation, the possibility of achieving long-term shunt independence with ETV should be considered preferable in good-risk patients when compared with the cumulative morbidity of multiple shunt procedures.Reference Brockmeyer, Abtin, Carey and Walker 67 Absolute contraindications to ETV include small ventricular size preventing safe intraventricular manipulation of the scope and a tumor obstructing the surgeon's access to the floor of the third ventricle.Reference Rekate 69 These scenarios aside, the difficulties inherent to pediatric endoscopy and the risks associated with more complex cases may be alleviated with the use of neuronavigation, a technique that has been shown to a safe and beneficial adjunct to endoscopy.Reference McMillen, Vonau and Wood 70 - Reference Rohde, Behm, Ludwig and Wachter 72 Several systems, such as AxiEM (Medtronic, USA) currently used at our center, allow frameless navigation of the endoscope in patients that are unable to safely undergo rigid cranial fixation in pins because of young age or thin skull vaults, and in the subset of patients who have complicated or distorted ventricular anatomy.Reference McMillen, Vonau and Wood 70 , Reference Choi, Seo, Kim, Kim, Kim and Lee 73
Tumors
Endoscopic tumor management is a safe and effective alternative to conventional means and allows the option of ETV or septostomy for treatment of associated hydrocephalus.Reference Najjar, Azzam, Baghdadi, Turkmani and Skaf 74 - Reference Wong, Chen, Liang, Yen and Chang 90 The results in most series are based on data from both adult and pediatric populations. Neuroendoscopy in the field of neurooncology offers the advantages of improved visualization of intraventricular pathology, better management of tumor-related hydrocephalus, less morbid biopsies, and the allowance of minimally invasive removal of intraventricular tumors.Reference Badie, Brooks and Souweidane 77 , Reference Oppido, Fiorindi and Benvenuti 91 Certain characteristics make a tumor ideally suited for endoscopic biopsy or resection.Reference Teo and Nakaji 78 These include moderate to low vascularity, soft-consistency, tumors smaller than 2 cm in diameter, those associated with secondary hydrocephalus, those that are histologically low grade, and lesions situated in the lateral ventricle and pineal region.Reference Hanada, Oyoshi, Hirano and Arita 76 , Reference Tirakotai, Riegel and Stiegel 92 In addition, tumors that will be treated primarily nonsurgically, such as gliomas of the tectal region, are ideally suited for primary treatment of obstructive hydrocephalus and, if appropriate, neuroendoscopic biopsy.Reference Badie, Brooks and Souweidane 77
In our series, 27 intraventricular tumors were biopsied, four of which were also resected. Of the 27 patients, there were 20 adults and seven pediatric patients. The overall major complication rate was 3.7%, which is consistent with that of other series. Constantini et al reported a large series of 293 patients who underwent endoscopic tumor biopsy with an infection rate of 3% and a mortality rate of 0.3%.Reference Constantini, Mohanty and Zymberg 93 Another report by Luther et al of 86 intraventricular tumors that were biopsied or resected had a hemorrhagic sequelae rate of 3.5%.Reference Luther, Cohen and Souweidane 79 They defined clinically significant hemorrhage as that resulting in abandonment of the procedure, postoperative hydrocephalus requiring shunting, neurological change, or hemorrhage that required a second procedure to treat the bleed. In addition, Tirakotai et al described 46 patients (mean age 43.8 years) with peri- and intraventricular tumors who underwent neuroendoscopic procedures with three transient morbidities and one permanent deficit, but no operative mortality.Reference Tirakotai, Hellwig, Bertalanffy and Riegel 81
Arachnoid Cysts
Primary arachnoid cysts are developmental lesions containing a fluid similar to CSF and are often incidentally found in patients as a focal area of fluid accumulation with bulging and thinning of the adjacent skull.Reference Awaji, Okamoto and Nishiyama 94 Overall, arachnoid cysts account for approximately 1% of all intracranial mass lesions, with potentially up to 60-90% of these becoming symptomatic in childhood.Reference Pradilla and Jallo 95 Patients presenting with symptoms or signs of increased intracranial pressure or seizures and with growing arachnoid cysts are typically considered for surgical treatment. Endoscopic surgery has become the treatment of choice when feasible because of its minimal invasiveness and good outcomes.Reference Pradilla and Jallo 95 - Reference Karabagli and Etus 105 Assessment of the anatomical relationship between the arachnoid cyst wall and adjacent anatomical structures such as blood vessels, cranial nerves, and brain parenchyma is necessary during preoperative planning to avoid injury to these structures. Complications may include intraoperative bleeding obscuring the surgeon's visual field, postoperative subdural hematomas or hygromas, meningitis, or the injuring of adjacent structures during fenestration.Reference Abbott 106
In our combined series, 53 cyst fenestrations were performed, 16 in adults and 37 in pediatric patients. The overall major complication rate was 7.5%, which is similar to that of other series. For example, Nowoslawska et al reviewed 106 children with arachnoid cysts, 44 of whom underwent a neuroendoscopic procedure and 62 of whom underwent other operations.Reference Nowoslawska, Polis and Kaniewska 101 Eleven of the 44 experienced complications, including five CSF leaks, two third nerve palsies, two subdural hematomas, one case of meningitis, and one case of severe intraoperative bleeding. In the nonendoscopic group, there were ten with complications. In a smaller series reported by Huang et al of 15 patients who underwent neuroendoscopic arachnoid cyst fenestration, there was only one complication of chronic subdural hematoma that was treated with burr hole drainage.Reference Huang, Wang, Guo, Zhou, Wang and Li 100 The authors concluded that, compared with traditional treatments, neuroendoscopic cystic fenestration is more effective and minimally invasive with less mortality and morbidity. Similarly, two additional patient series, one reported by Schroeder et al of 21 endoscopically fenestrated arachnoid cysts revealed no deaths or permanent complicationsReference Schroeder, Oertel and Gaab 102 ; another study by Pradilla et al of 11 endoscopically treated arachnoid cysts (mean age 10.9 years) found one patient left with persistent spasticity and one patient suffering mild hemiparesis.Reference Pradilla and Jallo 95 These authors concluded that cyst fenestration is favored over shunting as the method of choice for initial cyst decompression.
Colloid Cysts
Colloid cysts are benign space-occupying lesions that typically arise in the region of the anterior third ventricleReference Charalampaki, Filippi, Welschehold and Perneczky 107 and represent about 0.5-2% of intracranial tumors.Reference Grondin, Hader, MacRae and Hamilton 108 Although uncommon, they are an important clinical entity because of their ability to cause CSF obstruction at the foramina of Munro with resultant hydrocephalus, which in the acute state can lead to sudden death.Reference Grondin, Hader, MacRae and Hamilton 108 If the obstruction of the foramina of Monro is asymmetrical, one lateral ventricle can grow larger than the other, resulting in bowing of the septum pellucidum to the nonaffected side.Reference Charalampaki, Filippi, Welschehold and Conrad 109 Although microsurgery has been the classic treatment option,Reference Longatti, Godano and Gangemi 110 the composition and location of these cysts make them ideally suited for endoscopic excision.Reference Mishra, Chandra, Suri, Rajender, Sharma and Mahapatra 111 - Reference Margetis and Souweidane 115 Expectedly, the success of endoscopic colloid cyst removal is heavily dependent upon the surgeon’s experience.Reference Badie, Brooks and Souweidane 77
In our series, 32 colloid cysts were endoscopically resected, 31 of which were in adult patients. The overall major complication rate was 3.1%, and the cyst recurred in one case. We have previously presented our data in comparison to microsurgical resections, showing that endoscopic resection of colloid cysts can be performed with a significantly lower risk of complications than microsurgical resection (4% vs 33%), and with equivalent surgical success.Reference Grondin, Hader, MacRae and Hamilton 108 In addition, operative time and length of hospital stay are both significantly reduced with endoscopic resection. Similar results were found in a series of 30 patients reported by Lewis et al,Reference Lewis, Crone, Taha, van Loveren, Yeh and Tew 116 where half underwent open resection and the other half endoscopic resection of a colloid cyst. They found the endoscopic group had shorter hospital stay and operative time, as well as a shorter interval before returning to work. Similarly, Longatti et al reported on 61 patients (mean age 41 years) undergoing endoscopic colloid cyst resection who had a complication rate of 3.2%, with seven asymptomatic recurrences,Reference Longatti, Godano and Gangemi 110 and Greenlee et al reported 35 patients (mean age 32.4 years) experiencing a complication rate of 8.6%.Reference Greenlee, Teo, Ghahreman and Kwok 117
Summary and Conclusions
Intracranial, intraventricular neuroendoscopy was performed in 161 adult and 112 pediatric patients. A higher percentage of pediatric patients underwent cyst fenestration, whereas a higher percentage of adults underwent ETV, colloid cyst removal, and tumor biopsy. The most common complication associated with neuroendoscopy was infection, and adult and pediatric patients had similar complication rates. These findings highlight the important potential role for neuroendoscopy during management of appropriate adult patients. Therefore, neuroendoscopy should be an available therapeutic modality in all neurosurgical centers and should be considered as a potential therapeutic modality in the management of suitable adult as well as pediatric patients.
Disclosures
All authors have seen and approved this manuscript and have no disclosures to report. No portion of this work has been previously published or presented. FG, RD, WH, and MH declare they have no conflict of interest.






