Introduction
Septic embolus is a complication often seen in patients with infective endocarditis (IE). In this subgroup, 20%–40% patients are at increased risk of developing neurological complications such as acute ischemic stroke (AIS), intracerebral haemorrhage and mycotic aneurysm.Reference Walker, Sampson, Skalabrin and Majersik1–Reference Morris, Matiello, Lyons and Samuels3 Mortality rates as high as 56% have been reported in patients with stroke secondary to septic emboli.Reference Walker, Sampson, Skalabrin and Majersik1, Reference Ruttmann, Willeit and Ulmer4
There is a lack of a defined pathway or consensus on the appropriate acute stage treatment strategy in patients with IE with large vessel occlusion (LVO).Reference Kim, Jeon, Kim, Kang, Hwang and Kim5 Systemic thrombolysis using intravenous tissue plasminogen activator (IV-tPA) may potentiate the risk of haemorrhage.Reference Walker, Sampson, Skalabrin and Majersik1, Reference Kim, Jeon, Kim, Kang, Hwang and Kim5 To this end, endovascular thrombectomy (EVT) is emerging as a potentially efficacious and safer intervention strategy in this subgroup of patients.Reference Liang, Bishu and Anavekar6, Reference Sveinsson, Herrman and Holmin7 We recently reported a case study on the usefulness of EVT and clot histopathology in a patient with stroke secondary to IE.Reference Bhaskar, Cordato and Cappelen-Smith8 EVT also offers further benefit to pursue a thorough histopathologic analysis including routine Gram staining of EVT-retrieved clots, which may provide additional diagnostic/etiologic information in non-IE patients,Reference Niesten, van der Schaaf and van Dam9 and in those with suspected IE or prosthetic valvular heart disease.Reference Boeckh-Behrens, Kleine and Zimmer10 This perspective was unfortunately missing from the recently published consensus guidelines of thrombus analysis in AIS.Reference De Meyer, Andersson and Baxter11 In this paper, we present case series of four consecutive IE patients who received EVT at our comprehensive stroke centre (CSC). EVT-retrieved clots were sent for comprehensive histopathological and morphological analysis. We present our findings with emphasis on clot morphology, which may offer a unique window for better diagnostic characterisation and effective secondary stroke treatment.
Materials and Methods
AIS patients with LVO aged >18 years admitted to our institution over a 12-month period (January 2017–January 2018) with IE who received EVT, which constituted 3.2% (4/126) of EVT-treated patients, were included in this study. All patients underwent diagnostic scanning using routine AIS imaging protocol comprising of non-contrast computed tomography (NCCT), spiral CT angiography (CTA) from the aortic arch to circle of Willis and neuro CT perfusion (CTP). Clinical diagnosis of IE was confirmed using the Duke diagnostic criteria following routine diagnostic work-up including blood cultures, haematological analysis and transesophageal echocardiography (TEE). All patients underwent EVT using aspiration or combined stent retriever and aspiration technique under general anaesthesia. Ethics approval for the study was obtained from the South-Western Sydney Local Health District Human Research Ethics Committee.
Liverpool Clot Histopathological Examination Protocol
Prior to this case series, there was no standardised histopathological assessment of the EVT-retrieved clots at our institution. It was found that retrospective staining was required for Gram stain (Case 2), and so a comprehensive histopathological clot analysis protocol was developed to routinely examine prospective EVT-retrieved clots (Cases 1, 3 and 4). All EVT-retrieved clots were fixed in formalin in adherence with normal septic handling procedures. Formalin-fixed clots were then reviewed for their colour, size and morphology. Routine histology using haematoxylin and eosin (H&E) stain and additional special stains were performed. For staining, formalin-fixed, paraffin-embedded (FFPE) tissue block specimens were prepared. The special stain protocol included Gram staining (to distinguish gram-positive and gram-negative bacteria), the Periodic Acid Schiff for Fungus Stain Kit (PASF), Gomori’s Methenamine-Silver Nitrate (GMS) stain (for screening fungal organisms), Masson Trichrome (for detection of collagen fibres in tissue sections), Von Kossa (for histologic visualisation of calcium deposits in tissue specimens), Verhoeff-Van Gieson elastic staining (EVG) (for detecting elastic fibres) and Perls’ Prussian Blue staining (for histological visualisation of hemosiderin/iron).
Results
Case Series
Case Study 1
An 80-year-old man was presented with septic shock to a primary stroke centre, with a systolic blood pressure (SBP) of 70 mmHg and sudden onset of right-sided hemiplegia and dysphagia. His past medical history included IE a year ago, mitral and atrial bioprosthetic valve replacements (M/AVR) and paroxysmal atrial fibrillation. TEE demonstrated vegetation on the anterior cusp of the transcatheter aortic valve implantation (TAVI) prostheses as well as an echo dense lesion on the atrial side of the A2 scallop of the mitral valve. The patient was in sinus rhythm during his subsequent transfer and admission to our CSC. He was febrile (temperature of 38.3°C) and had received empirical treatment for recurrent IE with Flucloxacillin, Vancomycin and Gentamicin. National Institute of Health Stroke Scale (NIHSS) at presentation was 16 and Glasgow Coma Scale (GCS) was 11. Baseline NCCT showed hyperdense left M1 segment of the middle cerebral artery (MCA) artery with loss of grey–white matter differentiation in the left insular region consistent with an acute cerebral infarction. CTA showed occlusion of the left internal carotid artery (ICA) extending to the M1 segment of the MCA. CTP showed recent left MCA territorial infarction with large surrounding penumbra as demonstrated by decreased cerebral blood flow (CBF), decreased cerebral blood volume (CBV), prolonged mean transit time (MTT) and prolonged transient time to peak (TTP) in left MCA territory. Due to haemodynamic instability, the patient was presented to our CSC after a considerable delay. Groin access was established at 5 h 35 min following the symptom onset. EVT achieved good reperfusion with a modified thrombolysis in cerebral infarction (mTICI) score of 2b at 7 h 45 min after the stroke onset. However, prerolandic cortical branch occlusion and proximal A1 occlusion of the anterior cerebral artery (ACA) persisted. Moderate sized area of infarction involving the left frontal and temporal lobes was observed on follow-up imaging following EVT. NIHSS at 24 h was 21.
The septic clot retrieved after EVT from the left ICA and M1 was sent for histopathology and Gram staining. The specimen consisted of three tan to dark brown fragments, with size varying from 2 to 28 mm in length and 2 mm in width. There were two morphologically distinct areas within the clot. The first, which occupied a quarter of the specimen, showed mostly acellular densely hyalinised fibrinoid material with essentially no blood and a few scattered neutrophils. Gram staining showed the presence of colonies of Gram-positive cocci in clusters in this material. The other three quarters of the specimen showed a fresh thrombus composed of blood and bands of fibrin mixed with variably sized empty vacuoles, presumed to be gas introduced at the time of the EVT procedure. The thin wispy bands of fibrin were associated with a few neutrophils and eosinophils, which tended to aggregate around the fibrin bands. No lymphocytes or cholesterol clefts were present. All other special stains were negative. Overall, these findings were consistent with a vegetation embolising from the heart in a background of IE and an associated fresh thrombus. The images of histopathological analysis for this case are shown in Figure 1(A)–(C).
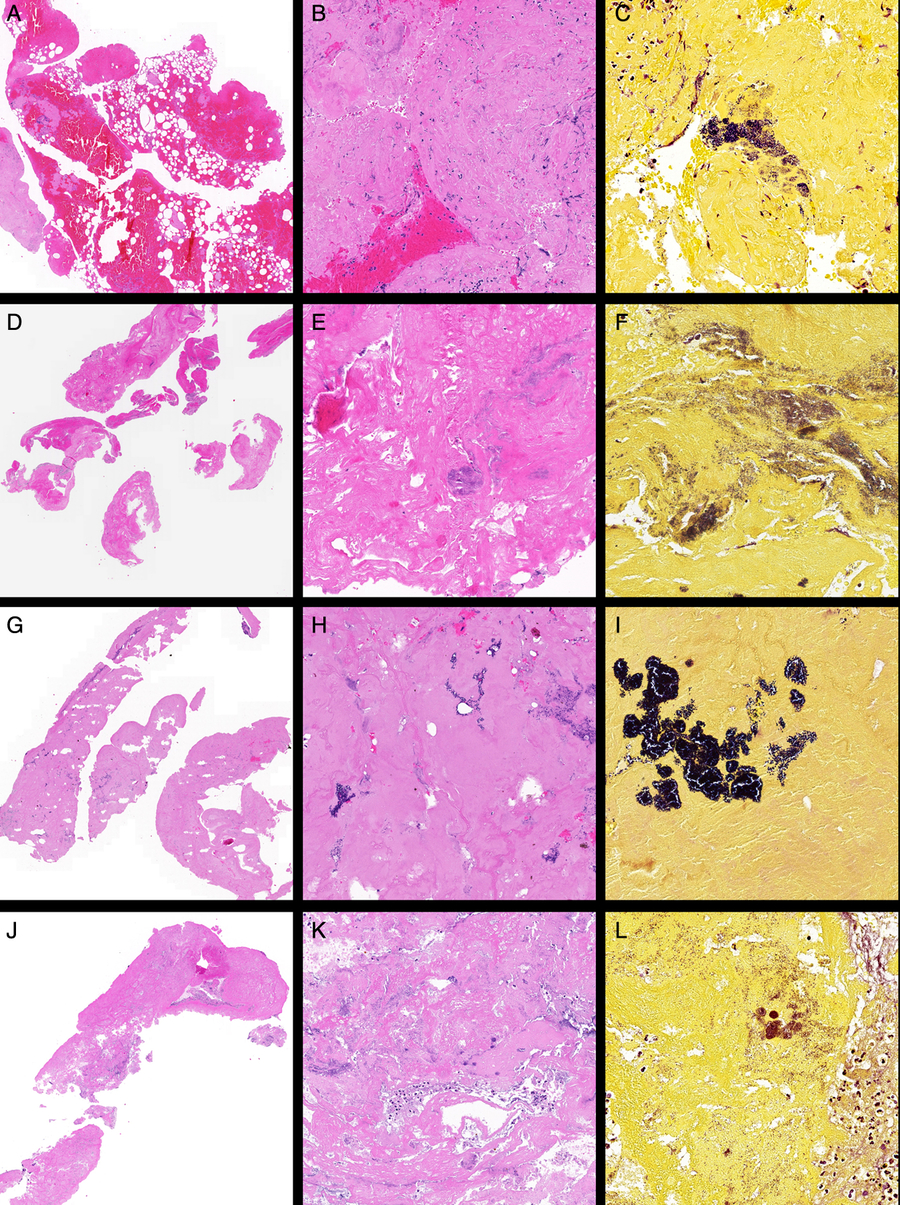
Figure 1: Representative histopathological images of the clots retrieved after EVT from patients with IE. Case 1: (A) Low-power view of fibrin-rich thromboembolus (left) with attached blood-rich thrombus (right) (H&E stain, ×2), (B) high-power view of paucicellular fibrin-rich thromboembolus (H&E stain, ×20) and (C) Gram stain showing colonies of Gram-positive cocci (×40). Case 2: (D) Low-power view (H&E, ×2), (E) high-power view of paucicellular fibrin-rich thromboembolus showing suspected colonies of bacteria (H&E stain, ×20) and (F) Gram stain (×40). Case 3: (G) Low-power view of fibrin-rich thromboembolus (H&E, ×2), (H) high-power view of paucicellular fibrin-rich thromboembolus showing colonies of bacteria (H&E stain, ×20) and (I) Gram stain (×40). Case 4: (J) Low-power view of fibrin-rich thromboembolus (H&E, ×2), (K) high-power view (H&E stain, ×20) and (L) Gram stain (×40).
Case Study 2
This case, of a 36-year-old man with a previous bioprosthetic aortic valve replacement (AVR) who developed an acute onset left hemiparesis whilst exercising, has previously been discussed in our recently published report.Reference Bhaskar, Cordato and Cappelen-Smith8 He was presented to our hospital following an episode of collapse and a baseline NIHSS of 16. CTA of the neck and circle of Willis revealed a right M1 occlusion. Patient received systemic thrombolysis and underwent EVT. EVT with aspiration achieved excellent reperfusion (mTICI score 3) at 3 h 30 min post-symptom onset. Routine histology using H&E stains showed a fibrin-rich thromboembolus with acute inflammatory cells. At this time, Gram stain was not routinely used for examination of EVT clots. Extensive investigation in hospital did not establish a cardiac source of embolism. His recovery profile was excellent with NIHSS at 24 h of 2. He was discharged home after 5 days with modified Rankin score (mRS) of 1 on daily regimen of metoprolol (25 mg) and aspirin (100 mg). The patient developed polyarthralgias without evident fever 3 weeks post-discharge. A large aortic valve vegetation was confirmed on repeat echocardiography. Routine histology using H&E stains showed fibrin-rich thromboembolus with acute inflammatory cells, and retrospective Gram staining of the initial clot confirmed septic embolism (see Figure 1(D)–(F)). Gram stain showed colonies of Gram-positive cocci. Follow-up MRI showed a right MCA territory subacute infarct with mass effect and midline shift.
Case Study 3
A 91-year-old man was presented with dense left-sided hemiparesis and slurred speech. He had a past history of ischemic heart disease (IHD) and aortic bioprosthetic valve replacement for aortic stenosis, clinically recurrent IE (previously treated with complete course of intravenous antibiotics for 6 weeks), hypertension, dyslipidaemia, severe mitral annular calcification with mild-to-moderate mitral stenosis and right upper lung lobe and colonic adenocarcinoma (both resected more than 5 years ago). Echocardiography revealed prosthetic aortic valve thrombosis suggestive of the valve vegetation. Patient was clinically diagnosed as having IE in accordance with the Duke criteria. NIHSS at admission was 19. Baseline NCCT imaging showed high density in the right M1 segment of the MCA without evidence of established infarction. CTA confirmed abrupt cut-off with non-filling of the distal right M1 segment of the MCA (see Figure 2). Moderate-grade calcific stenosis was observed on the proximal ICA bilaterally and high-grade stenosis was also seen on the left intracranial portion of the vertebral artery. Patient received tPA thrombolysis followed by EVT. EVT achieved excellent reperfusion with mTICI 3 score at 3 h 30 min after symptom onset (Figure 2(D)). Neurological improvement was noted following the procedure. The images pertaining to this case are presented in Figure 1. Magnetic resonance imaging (MRI) showed a subacute right MCA territory infarct with mass effect and mild midline shift. Follow-up MR angiography showed no evidence of mycotic aneurysm. Blood cultures grew Enterococcus faecalis confirming recurrence of IE. The patient was managed with ampicillin, gentamycin and amoxicillin. His 24-h NIHSS was 11. He was discharged from a hospital with an mRS score of 4.
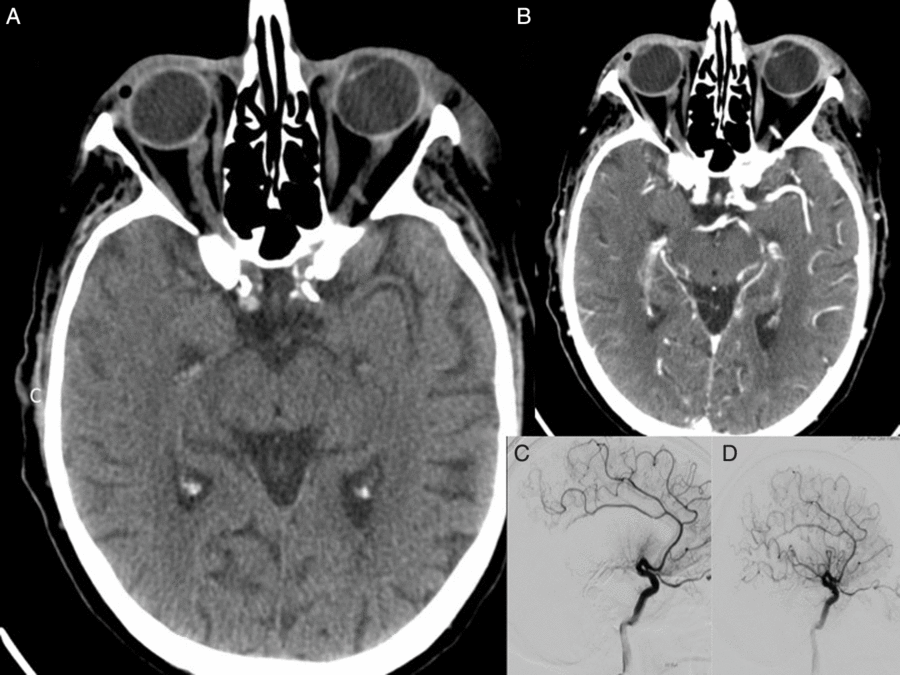
Figure 2: Pre- and post- EVT imaging for Case Study 3. Baseline NCCT showed hyperdense distal right M1 segment in the right MCA territory consistent with the evolving infarction (A). CT angiogram of the circle of Willis revealed abrupt cut-off at the distal M1 segment of the right MCA (B). Cerebral DSA confirmed mid-right M1 occlusion (C). Patient received EVT using single pass with Solitaire 4 × 20 mm and aspiration via a Sofia Plus 6F. Following EVT, patient showed excellent angiographic reperfusion with mTICI score of 3 (D). The follow-up, post-EVT, CT scan showed acute infarction in the right frontal temporal cortex and external capsule. Subacute infarction in the right occipital cortex was also noted.
Histopathological analysis was performed on the retrieved clot specimen (Figure 1(G)–(I)). The clot consisted of six fragments of 1–10 mm in length. Microscopic examination revealed small fragments of embolus comprising of blood, fibrin and aggregates of neutrophils. Colonies of both Gram-positive and Gram-negative cocci, possibly from cardiac valve consistent with IE, were observed on Gram stain in the fibrin-rich areas of the clot (Figure 1(I)).
Case Study 4
A 71-year-old female with history of coronary artery bypass graft (CABG) for dual vessel disease and metallic aortic valve replacement (AVR), paroxysmal atrial fibrillation (on warfarin regimen), hypertension and hypercholesterolemia presented with left-sided upper limb weakness on a background of methicillin-resistant Staphylococcus aureus (MRSA) sepsis. Echocardiography revealed the presence of vegetation on the metallic aortic valve. Her baseline NIHSS was 26.
Baseline NCCT revealed loss of grey/white matter differentiation in the right MCA territory consistent with a right MCA stroke. CTA confirmed complete occlusion of the right ICA extending from its proximal portion (approximately 15 mm distal to its origin) to its termination. Minimal flow was also observed in the right M1/proximal M2 with the absence of distal flow. There was crossover flow from the left ICA via the anterior communicating artery into the right ACA. CTP demonstrated an established ischemic core and large penumbra in the right MCA territory. EVT with combined stent retriever and aspiration achieved excellent mTICI 3 reperfusion at 6 h 10 min after the stroke onset. NIHSS score at 24 h was 12.
Clot retrieved from the right ICA and MCA was sent for histopathological analysis. The clot consisted of two fragments, approximately of 1-mm width each, and 5 and 30 mm length, respectively. Macroscopic examination of the whole clot showed pinkish-red soft tissue appearance. Microscopy showed dense fibrinoid material mixed with clusters of neutrophils with no evidence of fresh blood (Figure 1(J) and (K)). Routine histology showed numerous colonies of bacteria scattered throughout the clot. Gram staining demonstrated the presence of numerous coccal organisms including some round-shaped Gram-positive bacteria, confirming the earlier positive blood cultures indicative of S. aureus (Figure 1(L)). No lymphocytes or cholesterol clefts were observed. However, Von Kossa and Perl’s Prussian Blue staining revealed the presence of small nodule of calcification and scattered granules of hemosiderin, respectively. EVG staining for the elastic fibres was negative. Interestingly, collagen-rich soft tissue was seen within the fibrinoid material (on Masson Trichrome staining), presumably small fragments of the vessel wall.
Discussion
EVT, with or without systemic thrombolysis, has revolutionised stroke treatment and is being increasingly adopted as the standard of acute stroke treatment.Reference Bhaskar, Stanwell, Cordato, Attia and Levi12 However, this highly specialised intervention can be only offered to much selected group of patients with favourable pre-treatment CT imaging profiles. These too must be obtained within a constrained time window of 4.5 h since stroke onset for intravenous thrombolysis and 6 h and up to 24 h for EVT in selected patients.Reference Nogueira, Jadhav and Haussen13 Therefore, only a few patients with stroke and IE are likely to receive this intervention, but this will change as time progresses.
Our case series is therefore exceedingly rare.Reference Hernandez-Fernandez, Rojas-Bartolome and Garcia-Garcia14 A recent study on histopathological and bacteriological analyses of EVT-retrieved IE clots by Hernandez-Fernandez et al. reported an increased proportion of septic emboli diagnosis.Reference Hernandez-Fernandez, Rojas-Bartolome and Garcia-Garcia14 Stroke patients with IE are most likely to have very poor prognosis. In the milieu of EVT, we now have a rare opportunity to study the clot profiles and see its value in management of stroke patients. In our series of four cases, the novel finding was the unique brain clot morphology of paucicellular fibrin bands around vegetation which may be commonly seen by cardiovascular pathologists, but have not been reported in extracted brain clots – to the best of our knowledge. The composition of these clots is different from non-septic embolic clots.Reference Hernandez-Fernandez, Rojas-Bartolome and Garcia-Garcia14, Reference Brinjikji, Duffy and Burrows15 Stroke patients with IE often respond very poorly to reperfusion intervention including IV-tPA and till date we have very limited understanding of why these patients respond as they do. In our case series, patients responded favourably to EVT.
This study on a case series presents an account of the value of clot analysis and EVT in patients with suspected IE and valvular heart disease and artificial or bioprosthetic valve replacements. It also presents a detailed characterisation of the EVT-retrieved clots in these patients using comprehensive histopathological and morphological analysis.
Histopathological and Gram staining may have a value in routine pathology work-up to confirm clinical diagnoses and as an adjunct decision-making tool for prompt treatment planning in acute stroke settings. Gram staining of the clots was able to confirm the diagnosis of septic embolism due to IE and classify the clot as a septic embolus. A consistent observation in our case series was the presence of dense paucicellular fibrinoid material mixed with clusters of bacterial cocci. This morphology is remarkably unique to septic emboli and may be useful in differential diagnoses of IE over incidental bacteraemia. However, differential features for IE and incidental bacteraemia need further research. To our knowledge, this is the first study describing the presence of this characteristic morphology using detailed histopathological and morphological analysis of EVT-retrieved clots including Gram staining, which may be of value in early diagnostics and treatment planning in IE.
The interspersion of red blood cells (RBCs) within bands of fibrin is probably due to in situ formation of fresh clot around the embolus. In the milieu of septic embolisation, IV-tPA has been reported to be associated with increased risk of bleeding and poor lytic efficacy – hence increased probability of poor angiographic and clinical outcomes.Reference Kim, Jeon, Kim, Kang, Hwang and Kim5, Reference Brownlee, Anderson and Barber16 Consistent with the predominance of RBCs interspersed within fibrin bands in our retrieved clots, we found prominent early imaging signs such as the hyperdense middle cerebral artery (HDMCA) sign on NCCT in all four cases. Other studies have also reported a correlation between these early imaging signs with the density of RBCs in the retrieved thrombi of non-IE-related cases.Reference Brinjikji, Duffy and Burrows15, Reference Kim, Yoon, Kim, Kim, Heo and Park17–Reference Liebeskind, Sanossian and Yong20
From a therapeutic perspective, it is advisable to initiate aggressive antibiotic therapy as early as possible in patients suspected with IE or with associated risk factors. This may potentially thwart further septic embolisation and cerebral infarction.Reference Kim, Jeon, Kim, Kang, Hwang and Kim5 Management of ischemic stroke patients with LVO and IE is clinically challenging due to lack of specific guidelines. It has been observed that the risk of embolisation may be higher in patients with large (>10 mm), left-sided mitral valve vegetation.Reference Kim, Jeon, Kim, Kang, Hwang and Kim5
Given that the diagnosis of IE is often delayed, and in some cases missed, there is an inherently increased risk of morbidity and mortality for the patient. Detailed clot histopathology including Gram staining should be undertaken in patients at heightened risks of IE such as those with native or prosthetic valvular heart disease. Clot morphology may be useful in distinguishing septic emboli from incidental bacteraemia. We acknowledge that septic emboli are not necessarily synonymous with IE. The clot analyses in all our four cases supported the clinical diagnoses of IE, and the emboli in these cases were pathological vegetations, thereby fulfilling the Duke criteria.
Limitations
The findings of this report should be appreciated within the context of this study population. Methodological limitations of this study include small sample size and lack of control cases, such as those with incidental bacteraemia, for comparison. Since the clot analysis confirmed clinical diagnoses of IE in this case series, the clot analysis including Gram staining did not necessarily change the treatment course. However, it is still useful in detailed characterisation and classification of the EVT-retrieved clots.
Conclusions
Understanding the composition and morphology of clots may offer a robust explanation on why patients with IE respond poorly to thrombolysis and carry increased risk of poor outcomes. We hypothesise, based on current findings, that variation in clot composition revealed by comprehensive histopathological examination may be useful in etiological characterisation.
Acknowledgements
We would like to thank our staff members from the Department of Pathology at Liverpool Hospital for their feedback and ongoing discussions around clot analysis.
Disclosures
The authors have no conflicts of interest to declare.
Statement of Authorship
SB, JS, MK and DC conceptualised the study. SB, JS, XJW and MK developed the histopathological protocol. SB wrote the first draft of the manuscript. All authors contributed to the revision, overall discussion and conduct of the study.




