Multiple sclerosis (MS) is a progressively disabling disease of the central nervous system. Symptoms may include impaired vision, weakness, ataxia, fatigue, bowel and bladder dysfunction, and cognitive impairment, among other symptoms.Reference Ewing and Bernard 1 Given the physical and cognitive impairments associated with MS, long-term care services may be needed to assist individuals with MS in maintaining their physical and mental well-being. Long-term care refers to a continuum of services that may be delivered in community (e.g. home care, day programs) or institutional (e.g. nursing home care) settings.Reference Northrop and Frankel 2 , Reference Northrop and Frankel 3
In the United States, several studies have evaluated the clinical and demographic characteristics of persons with MS admitted to Medicare- and Medicaid-certified nursing homes.Reference Buchanan 4 - Reference Buchanan, Martin, Wang and Ju 6 Compared with other nursing home residents, persons with MS were younger at admission and more physically disabled, but less cognitively impaired.Reference Buchanan 4 Depression was common at admission and it increased substantially in the year after admission, yet most persons with MS did not receive mental health services.Reference Buchanan, Wang, Tai-Seale and Ju 5 , Reference Buchanan, Martin, Wang and Ju 6 These findings raise questions about the quality of care for persons with MS in these settings, both in the availability of mental health services and providers’ capacity to respond to the psychosocial needs of this patient population. Further, it is uncertain how persons with MS who receive care in institutional settings may differ from those who receive long-term care services in the community, a question of importance to patients, their families, and policy makers.
In Canada, interRAI Resident Assessment Instrument (RAI) assessments are used as part of routine clinical practice in home care, nursing home, Complex Continuing Care (CCC) hospitals, and inpatient psychiatry. Data from these assessments populate the Continuing Care Reporting System, Ontario Association of Community Care Access Centres Home Care Database, and the Ontario Mental Health Reporting System.Reference Hirdes, Mitchell, Maxwell and White 7 - 10 The interRAI assessment system includes a suite of comprehensive clinical assessments that are compatible across health care settings to collect person-level data in domains such as physical functioning, cognition, mood and behavior, social functioning, disease and health conditions, health service, and medication utilization.Reference Gray, Berg and Fries 11 - Reference Barnaba, Landi, Onder, Liperoti and Gambassi 13 Widespread implementation of interRAI assessments in CanadaReference Hirdes, Mitchell, Maxwell and White 7 , Reference Hirdes 8 provides an opportunity to compare the clinical characteristics of persons with MS across multiple care settings. Substantial international adoption of interRAI assessments also permits national comparisons of clinical features and care provision for persons with MS.Reference Barnaba, Landi, Onder, Liperoti and Gambassi 13
The Innovations in Data, Evidence and Applications for Persons with Neurological Conditions project was conducted to estimate the cross-sector prevalence, clinical characteristics, and needs of persons with eleven neurological conditions living in Canada. The present study provides prevalence estimates and a clinical profile of individuals affected by MS across four care settings: inpatient mental health, home care, nursing home, and CCC hospitals.
Methods
Data Sources
Continuing Care Reporting System
Nursing homes and CCC hospitals in nine Canadian provinces and territories have implemented the RAI Minimum Data Set (RAI-MDS 2.0) or its successor the interRAI Long Term Care Facility assessment (being implemented in New Brunswick). All individuals with a length of stay of 14 days or longer are assessed with the RAI-MDS 2.0, providing near census-level health information on individuals within these care settings. Implementation dates differ by province, and two (Alberta and New Brunswick) had not begun to submit data to the Canadian Institute of Health Information at the time of this study. The nursing home cohort comprises the most recent assessments for unique individuals completed from July 1, 2003, to March 31, 2011, in Ontario and Nova Scotia; July 1, 2006, to March 31, 2011, in British Columbia; and July 1, 2008, to March 1, 2011, in Manitoba (Winnipeg Regional Health Authority only, accounting for 60% of the Manitoba population), Newfoundland, Saskatchewan, and Yukon Territory (Table 1). CCCs provide hospital-based long-term complex medical care, geriatric assessment and rehabilitation, psycho-geriatric care, palliative care, and respite care.Reference Teare, Daniel, Markel, McKillop, Pink and Rashkovan 14 They are differentiated from nursing homes in Ontario based on their delivery of care to medically complex patients as opposed to frail persons with stable medical conditions.Reference Hirdes, Mitchell, Maxwell and White 7 The CCC cohort includes the most recent assessments from April 10, 1996, to March 31, 2011, in Ontario, and from July 1, 2008, to March 31, 2011, in Manitoba (Table 1).
Table 1 Assessment period coverage by care setting cohort and Canadian province/territory
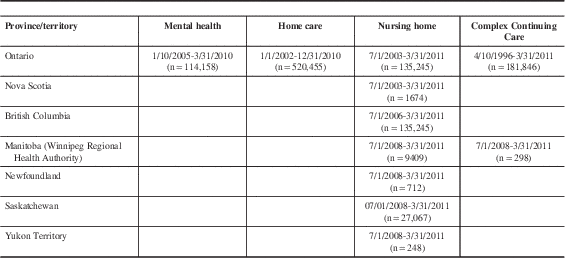
Ontario Association of Community Care Access Centres Home Care Database
Individuals who are expected to use home care services provided by one of Ontario’s 14 Community Care Access Centres for 60 days or longer, representing one-third of clients, are evaluated using the RAI-Home Care (RAI-HC). 15 The home care cohort includes the most recent assessments for unique individuals from January 1, 2002, to December 31, 2010 (Table 1). Although RAI-HC data are available for other provinces, only the Ontario data were used because it was the only province to include home care data on all neurological conditions of interest for the larger project for which this study was conducted.
Ontario Mental Health Reporting System
All Ontario inpatient mental health patients across 55 hospitals and 13 specialty psychiatric facilities are assessed using the RAI-Mental Health (RAI-MH).Reference Hirdes, Smith and Rabinowitz 16 The mental health cohort includes the most recent admission assessments for unique individuals hospitalized from October 1, 2005, to March 21, 2010 (Table 1).
Clinical Scales and Items
The RAI-MDS 2.0, RAI-HC, and RAI-MH instruments share a common set of items and validated clinical scales to assess patients across a broad range of health domains, including physical functioning, cognition, mood and behavior, social functioning, diseases and conditions, health service, and medication utilization.Reference Gray, Berg and Fries 11 , Reference Barnaba, Landi, Onder, Liperoti and Gambassi 13 The Cognitive Performance Scale (CPS) is a measure of cognitive impairment that ranges from 0 (intact) to 6 (very severely impaired).Reference Morris, Fries and Mehr 17 - Reference Jones, Perlman, Hirdes and Scott 19 The Depression Rating Scale (DRS) is a depression screening instrument derived from seven mood items. The DRS ranges from 0 to 14; scores of 3 or greater indicate depressive disorders.Reference Burrows, Morris, Simon, Hirdes and Phillips 20 The Activities of Daily Living Hierarchy Scale is a measure of functional performance that ranges from 0 (no impairment) to 6 (total dependence) based upon the ability to complete early and late loss activities of daily living (ADLs).Reference Morris, Fries and Morris 21 The pain scale ranges from 0 (no pain) to 3 (excruciating pain) and is a highly predictive of visual analog scale pain scores.Reference Fries, Simon, Morris, Flodstrom and Bookstein 22 Finally, the Changes in Health, End-Stage Disease, Signs and Symptoms Scale is a measure of health instability that is predictive of mortality and ranges from 0 (no health instability) to 5 (very high health instability).Reference Hirdes, Frijters and Teare 23 . Reference Hirdes, Poss, Mitchell, Korngut and Heckman 24
Despite substantial overlap across interRAI instruments, it is important to note that the RAI-MH differs from the RAI-MDS 2.0 and RAI-HC instruments in its collection of information on appetite, pressure ulcers, and psychotropic drug use. For this reason, results on these items are not presented for patients in the mental health cohort.
Identification of Persons with MS
Persons with an MS diagnosis were identified based on pick list item responses on the RAI-MDS 2.0, RAI-HC, and free-text International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, Canada (ICD-10-CA) fields on the RAI-MH instrument. Clinicians are instructed to complete these assessments using all sources of information available. Although most information may be obtained through patient interview and observation, medical records (i.e. physician orders, laboratory data, medication records, and care plans), family members, and the attending physician may also be consulted to complete the assessment. Previous work has established the validity of these methods for identifying persons with an MS diagnosis compared with diagnoses listed on administrative hospital records.Reference Foebel, Hirdes, Heckman, Kergoat, Patten and Marrie 25 , Reference Lix, Yan, Blackburn, Hu, Schneider-Lindner and Teare 26 Instruments relying on pick list responses achieved very high sensitivity (90%-94%), specificity (99%-100%), and interrater agreement (kappa=0.76-0.84).Reference Foebel, Hirdes, Heckman, Kergoat, Patten and Marrie 25 The RAI-MH, which relies on ICD-10-CA code responses, also performed well with a sensitivity of 77%, specificity of 100%, and kappa of 0.61.Reference Foebel, Hirdes, Heckman, Kergoat, Patten and Marrie 25 MS prevalence estimates in Canada using the entire history of patient administrative health records were found to be comparable to estimates based RAI MDS 2.0, RAI-HC, and RAI-MH index assessments alone.Reference Danila, Hirdes and Maxwell 27
Comparison Groups
Four groups were identified for comparison in this study: MS; Alzheimer’s disease and related dementias (ADRD); other neurological conditions; and non-neurological conditions. The ADRD group comprised individuals identified with an ADRD diagnosis using the pick list and ICD-10-CA methods previously detailed for those with MS. The other neurological conditions group included individuals identified using interRAI assessments as having a diagnosis of one or more priority conditions included in the Public Health Agency of Canada’s National Population Health Study of Neurological Conditions, except ADRD.Reference Caesar-Chavannes and MacDonald 28 These diagnoses were Parkinson’s disease, epilepsy, traumatic brain injury, Huntington’s disease, spinal cord injury, amyotrophic lateral sclerosis, muscular dystrophy, cerebral palsy, and stroke. 29
Finally, individuals without a diagnosis of MS, ADRD, or any of the previously forementioned neurological conditions formed the non-neurological condition comparison group. Note that several conditions including congenital neurological deficit, neuropathy, migraine, tension headache, and other neurological conditions are not included in interRAI assessments, so individuals with these conditions may appear in any of the four study groups. With the exception of the non-neurological conditions group, group assignment was not mutually exclusive. Individuals with comorbid neurological conditions, including MS and ADRD, may appear in multiple groups. Table 2 details the composition of the other neurological conditions comparison group.
Table 2 Composition of the other neurological conditions comparison group across care setting cohorts
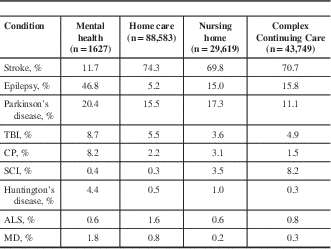
ALS=amyotrophic lateral sclerosis; CP=cerebral palsy; MD=muscular dystrophy; SCI=spinal cord injury; TBI=traumatic brain injury.
Analysis
Demographic and clinical characteristics of persons with MS, ADRD, other neurological conditions, and non-neurological conditions were compared within and across care setting using items and scales in the RAI-MDS 2.0, RAI-HC, and RAI-MH instruments. Chi-square tests were performed both between groups within each of the four care setting cohorts and within groups between the four care setting cohorts to ascertain the statistical significance of group and setting frequency differences. Given the large number of statistical tests performed, a Bonferroni correction was made.Reference Benjamini and Hochberg 30 This yielded an adjusted alpha of 0.05∕105=0.0005 per test. In part because of the large sample sizes used in this study, only demographic and clinical characteristic comparisons that were significant to an alpha level of 0.0001 were reported. In all care settings, the most recent assessment for each individual was included in the sample used to calculate group demographic and clinical characteristics.
To estimate the point prevalence of MS in each of the four care settings, a prevalence sample comprising Ontario patients receiving care in one of the four care settings on July 1, 2009 (index date) was created. This index date was selected because there was complete interRAI assessment coverage across the four care settings in Ontario at that time. For patients in nursing homes, CCC hospitals, and mental health settings, facility admission and all-cause discharge dates were used to determine inclusion in the prevalence sample. Home care patients in Ontario may remain on service for long periods without actively receiving home care services (e.g. home health aide or nursing visits). To restrict the prevalence sample to patients actively receiving home care services, only patients with a home care referral date before the index date and a RAI-HC assessment completed 180 days before or after the index date were included. Where all-cause discharge dates were available for home care patients, this information was also used to determine inclusion in the secondary sample. The number of individuals included in the denominator for each of the care settings was 110,123 in home care, 68,060 in nursing homes, 4945 in CCC hospitals, and 4360 in mental health settings.
All analyses were conducted using SAS, version 9.2, SAS Institute Inc., Cary, NC.
Results
Across the four care setting cohorts, 11,250 persons with MS were identified. In Ontario, among patients receiving care on July 1, 2009, MS was most prevalent in CCC hospitals (4125 cases per 100,000 patients (95% confidence interval [CI], 3596-4727), followed by home care (2020 cases per 100,000 patients [95% CI, 1939-2106]), nursing homes (1424 cases per 100,000 patients [95% CI, 1337-1516]), and mental health settings (138 cases per 100,000 patients [95% CI, 56-316]). In all four care settings, persons with MS were predominantly female and were younger than other persons in these settings (Table 3).
Table 3 Sociodemographic profile of persons with in the MS, ADRD, non-neurological and other neurological conditions comparison groups
Unless otherwise noted, the chi-square p values for group comparisons performed within and across settings are less than 0.0001.
Clinical Measures and Scales
Rates of moderate to severe cognitive impairment (CPS 3+) were higher in institutional settings than in the community. Fewer persons with MS in the home care and mental health cohorts had moderate to severe cognitive impairment compared with 39.6% in nursing homes and 42.3% in CCC hospitals (Table 4). Within all four care settings, persons with MS were less likely to have moderate to severe cognitive impairment (CPS 3+) compared with the ADRD and other neurological conditions comparison groups; however, their rates were consistently higher than in the non-neurological comparison group (Table 4).
Table 4 Clinical scale distributions for persons in the MS, ADRD, non-neurological, and other neurological conditions comparison groups
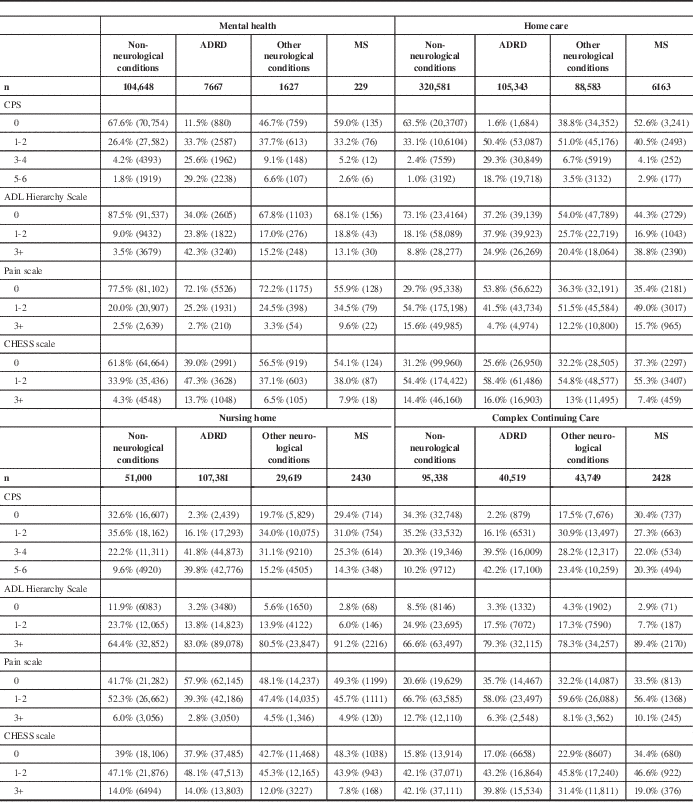
Unless otherwise noted, the chi-square p values for group comparisons performed within and across settings are less than 0.0001.
CHESS=Changes in Health, End-Stage Disease, Signs and Symptoms Scale; CPS=Cognitive Performance Scale.
Persons with MS in the nursing home and CCC cohorts were less independent in completing ADLs compared with those with MS in the home care and mental health cohorts. The percentage of patients with moderate to severe ADL impairment (Activities of Daily Living Hierarchy Scale 3+) was 13.1% of the mental health cohort and 38.8% of the home care cohort compared with 91.2% of the nursing home cohort and 89.4% of the CCC cohort (Table 4). Except for in mental health settings, persons with MS were more likely to be moderate to severely functionally impaired compared with the non-neurological conditions, ADRD, and other neurological conditions comparison groups in each setting (Table 4).
Mobility and Falls
Except in the mental health cohort, persons with MS were more likely to use a wheelchair than the ADRD, non-neurological conditions, and other neurological conditions comparison groups (Table 5). Irrespective of method of ambulation, 38.1% of persons with MS in home care fell in the 90 days before assessment. Fall assessment time frames differ on the RAI-MDS 2.0 and RAI-HC assessment. In the mental health cohort, 17.9% of persons with MS experienced a fall in the 30 days before assessment compared with 10.0% in CCC and 6.4% in nursing homes (Table 5). Within the mental health and home care setting cohorts, the rate of falls by persons with MS was similar to the ADRD and other neurological conditions comparison groups. However, within the nursing home and CCC cohorts, where they were likely to be wheelchair users, persons with MS were least likely of all groups to have experienced a recent fall (Table 5).
Table 5 Health and social profile persons with in the MS, ADRD, non-neurological, and other neurological conditions comparison groups
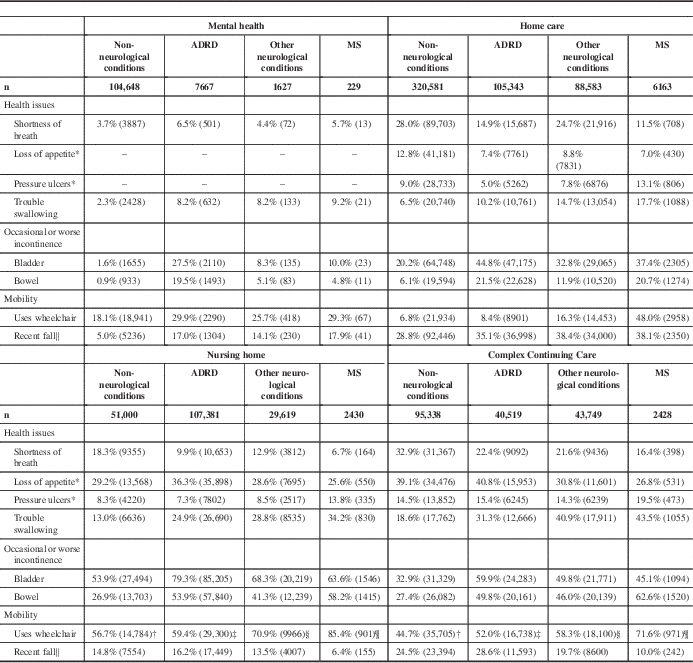
Unless otherwise noted, the chi-square p values for group comparisons performed within and across settings are less than 0.0001
* Items not available on RAI-MH assessment.
† Uses wheelchair item for patients in the non-neurological conditions group measured on 26,074 NH assessments and 79,965 CCC assessments.
‡ Uses wheelchair item for patients in the ADRD group measured on 49,368 NH assessments and 32,189 CCC assessments.
§ Uses wheelchair item for patients in the other neurological conditions group measured on 14,058 NH assessments and 31,021 CCC assessments.
¶ Uses wheelchair item for patients in the MS group measured on 1055 NH assessments and 1356 CCC assessments.
∥ Fell in past 30 days in MH/CCC/NH; fell in past 90 days in HC.
Mental Health Issues and Psychotropic Drug and Restraint Use
Across care settings, persons with MS were most likely to show signs of depression (DRS 3+) in the mental health (62.4%), nursing home (28.9%), and CCC (24.6%) care settings (Table 6). Within settings, persons with MS were generally more likely to show signs of depression compared with the other neurological conditions comparison group, but less likely to show signs of depression compared with the ADRD group (Table 6).
Table 6 Mental health, behavior, and psychotropic drug use profile of persons with in the MS, ADRD, non-neurological, and other neurological conditions comparison groups
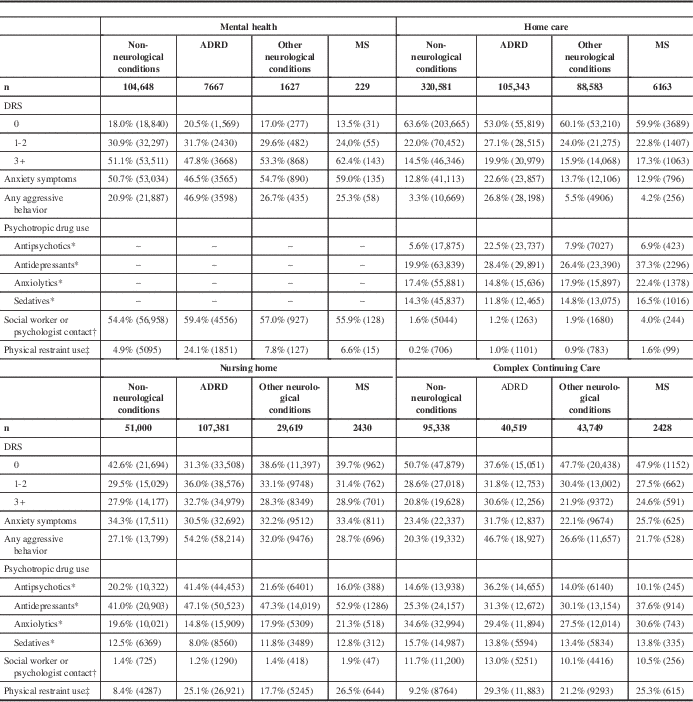
Unless otherwise noted, the chi-square p values for group comparisons performed within and across settings are less than 0.0001.
DRS=Depression Rating Scale
* Items not available on RAI-MH assessment.
† At least one 15-minute session in last 7 days; social worker in MH/HC, psychologist in MH/NH/CCC.
‡ Restraint use in the past 7 days in NH/CCC; restraint use in the past 3 days in MH/HC.
In the mental health cohort, 59.0% of persons with MS displayed anxiety symptoms compared with 12.9% in home care, 33.4% in nursing home, and 25.7% in CCC (Table 6). In the nursing home cohort, 28.7% of persons with MS displayed aggressive behaviors compared with 25.3% in mental health, 21.8% in CCC, and 4.2% in home care. Within all settings, persons with MS were less likely to have aggressive behaviors compared with the ADRD and other neurological conditions comparison groups (Table 6).
Except for the non-neurological conditions comparison group in the home care cohort, within care settings, persons with MS were least likely of all groups to be prescribed antipsychotic medications (Table 6). Conversely, within settings, persons with MS were most likely of all groups to be prescribed antidepressant medications. The same was true for anxiolytic and sedative medications, except in the CCC cohort (Table 6).
Care by a social worker or psychologist was infrequently provided outside of the mental health setting. Only persons with MS in the home care cohort were more likely than other groups to have contact with a social worker or psychologist on one or more occasions in the past 7 days (Table 6).
In the nursing home and CCC cohorts, a similar proportion of persons with MS were restrained with a mechanical restraint or chair that prevents rising in the 7 days preceding assessment (26.5% and 25.3%, respectively; Table 6). These rates are similar for the ADRD group in these two settings; however, in mental health only, 6.6% of persons with MS were restrained in the 3 days preceding assessment compared with 24.1% in the ADRD group (Table 6).
Discussion
This cross-sectional study establishes disease prevalence estimates and clinical profiles for persons with MS across the Canadian continuum of care. Using ADRD, other neurological conditions, and non-neurological conditions comparison groups, this study also differentiates persons with MS from other individuals within each of the care settings.
Several recent systematic reviews have summarized the epidemiological literature reporting the prevalence of MS among the general population in the North and South America,Reference Evans, Beland and Kulaga 31 Europe,Reference Kingwell, Marriott and Jetté 32 Africa, and Asia-PacificReference Makhani, Morrow and Fisk 33 regions. The current study aimed to estimate the prevalence of MS among individuals receiving care in community, institutional, and hospital-based care settings. Given that admission to these care settings is largely dependent on demonstrated need for formal care as a result of disability or illness, it is expected that the prevalence of MS in these care settings would be greater than among the general population. The prevalence of MS in the home care, nursing home, and CCC care settings in this study was approximately 7 to 21 times greater than in the Canadian general population, which ranges between 195 and 298 cases per 100,000 patients.Reference Kingwell, Zhu and Marrie 34 - Reference Beck, Metz, Svenson and Patten 38 The prevalence of MS among individuals in the mental health cohort was similar to that of the general population, likely because this care setting is oriented toward caring for patients with psychiatric conditions rather than physical disabilities. It has also been hypothesized that mental health facilities may not have the capacity to attend to the medical complexity of persons with MS,Reference Danila, Hirdes and Maxwell 27 thereby reducing access to psychiatric care for persons with MS who are more likely to have comorbid mental health conditions.Reference Marrie, Fisk and Yu 39
The results of this study illustrate that MS is a complex neurological condition that affects domains of health and well-being beyond physical and cognitive impairment. Most notable are mental health issues such as depression and anxiety. MS-specific instruments commonly used in clinical settings, such as the Expanded Disability Status Scale,Reference Kurtzke 40 focus primarily on the assessment of functional and cognitive impairment. These are included in interRAI assessments, in addition to numerous psychosocial, medical, and service use variables not considered by the Expanded Disability Status Scale. The assessment and management of these conditions has been identified as an important quality indicator in the delivery of care to persons with MS.Reference Cheng, Crandall and Bever 41
This study raises questions of the adequacy of psychosocial care outside of mental health care settings. It has been reported that nearly 40% of staff in Ontario CCC hospitals do not believe that they have the skills to address the mental health needs of patients.Reference Gibson, Kuluski and Lyons 42 Although psychotropic drug use was high among persons with MS, psychological therapy was rarely provided in home care, nursing home, and CCC settings. Despite evidence that psychological therapy is effective in treating depression among persons with MS,Reference Mohr, Boudewyn, Goodkin, Bostrom and Epstein 43 depressed persons with MS do not receive more psychological therapy than those without depression.Reference Buchanan, Wang, Tai-Seale and Ju 5 There may also be reason to question the capacity of mental health facilities to attend to the medical needs of persons with MS. Compared with individuals without neurological conditions, which account for 92% of psychiatric inpatients, persons with MS experience greater impairment in ADLs, incontinence, swallowing, and mobility. Recognizing the complex needs of persons with MS at various points along the continuum of care suggests that there is value in the use of comprehensive clinical assessments to guide care planning activities.
The results also demonstrate that there is considerable heterogeneity in the characteristics of persons with MS across care settings. Compared with those receiving home care, persons with MS in nursing homes and CCC hospitals were older and experienced greater physical and cognitive impairment. Given that MS is a progressive neurological condition, this variation is to be expected. In the general population, persons with MS had difficulties in similar domains such as ambulation, cognition, and pain.Reference Jones, Pohar, Warren, Turpin and Warren 44 Similar to the clinical profile of persons with MS in home care in this study, persons with MS in the general population report severe disability.Reference Jones, Pohar, Warren, Turpin and Warren 44 Given that MS affects numerous domains of health and wellbeing, comparisons of persons with MS between settings, jurisdictions, or countries should take into account the types of clinical covariates included in interRAI assessments.
Within care settings, the clinical profiles of persons with MS are distinct from individuals with other neurological and non-neurological conditions. Persons with MS in this study exceeded the level of functional impairment of peer groups in nursing home and CCC care settings; however, they are also a substantially younger patient population. Interviews with persons with MS in residential care settings suggest that they may find it difficult to interact with other residents with more severe cognitive impairment.Reference Riazi, Bradshaw and Playford 45 This presents a unique challenge in the delivery of person-centered care that is aligned with the individual strengths, preferences, and needs of minority patient populations in residential care settings. Organizational commitment to person-centered care may not be sufficient to ensure that the social needs of persons with MS are addressed given care providers’ orientation toward practice using a medical paradigm.Reference Rissanen, Ehrlich, Kendall and Muenchberger 46 Future research should aim to evaluate alternative models of care that promote quality of life among all residents, despite the inherent heterogeneity of nursing home residents.
Strengths and Limitations
The widespread implementation of interRAI instruments across the Canadian continuum of care provides population-level assessment data for persons with MS in several care settings and provinces. This creates a unique opportunity to conduct the largest study to date of persons with MS across the continuum of care in Canada. Using the interRAI suite of validated instruments enables cross-setting comparisons to be made of the clinical profile of persons with MS across four distinct care settings. Future work may take advantage of longitudinal nature of interRAI assessments to conduct analyses of the clinical trajectories of change in this population.
Unlike the new suite of interRAI assessments, the previous-generation assessments that were used in this study do not differentiate primary and secondary diagnoses. Thus, persons with MS who were included in this sample who have comorbid conditions may have clinical characteristics that are the results of MS. Although this might be seen as a limitation of using secondary data, it also provides a true representation of persons with MS receiving care in the respective care settings where comorbid conditions are common.Reference Marrie, Horwitz, Cutter, Tyry, Campagnolo and Vollmer 47 Comorbid conditions in this study were not differentiated because the assessments used in various settings contain different diagnoses lists. Also, despite validation of the diagnoses items used for case ascertainment in this study, Reference Foebel, Hirdes, Heckman, Kergoat, Patten and Marrie 25 - Reference Danila, Hirdes and Maxwell 27 it is possible that some individuals with an MS diagnosis may not be identified using these sources clinical health information. Finally, the interRAI assessments do not differentiate between subtypes of MS in their pick lists, and it is uncommon for additional information to be added on subtypes within the open-ended ICD diagnostic codes.
Conclusion
Persons with MS represent a small proportion of patients in each care setting across the continuum of care; however, the prevalence of MS in these community-, institutional-, and hospital-based care settings is high compared to in the general population. Despite considerable individual variability in MS symptomatology, through a comparison of interRAI assessment data for persons with MS in mental health, home care, nursing home, and CCC care settings, several setting-specific clinical profiles for persons with MS have emerged. Given the progressive nature of disability that is experienced by persons with MS, it is likely that over time persons with MS will receive care in one or more of the care settings that were included in this study’s analysis. The interRAI family of assessment instruments provides a valuable data source that can help to understand the characteristics of persons with MS across the continuum of care and to identify areas where care can be improved.
Acknowledgments and Funding
This study is part of the National Population Health Study of Neurological Conditions. We wish to acknowledge the membership of the Neurological Health Charities of Canada and the Public Health Agency of Canada for their contribution to the success of this initiative.
Funding for the study was provided by the Public Health Agency of Canada, Project #6271-15-2010/3970773. The opinions expressed in this publication are those of the authors/researchers, and do not necessarily reflect the official views of the Public Health Agency of Canada. RAM is funded in part by the Waugh Family Chair in Multiple Sclerosis. SBP is a Senior Health Scholar with Alberta Innovates, Health Solutions.
Disclosures
RAM receives research funding from: Canadian Institutes of Health Research, Public Health Agency of Canada, Manitoba Health Research Council, Health Sciences Centre Foundation, Multiple Sclerosis Society of Canada, Multiple Sclerosis Scientific Foundation, Rx & D Health Research Foundation, Consortium of MS Centers, and Crohn’s and Colitis Canada. RAM is the principal investigator for a Sanofi Clinical Trial; no personal compensation is received. JPH is a principal investigator for Public Health Agency/Canada and is a grant recipient. LAT and SBP have nothing to disclose.








