Introduction
Neuronal intranuclear inclusion disease (NIID) is a rare neurodegenerative disease that is characterized by eosinophilic hyaline intranuclear inclusions in neuronal and specific somatic cells. Reference Sone, Mori and Inagaki1 NIID was first documented as a rare disease featuring intranuclear inclusions in cells of the brain and visceral organs by Lindenberg in 1968. Reference Lindenberg, Rubinstein, Herman and Haydon2 According to the age of onset, NIID can be divided into three forms: the infantile form, juvenile form, and adult form. Both familial and sporadic cases have been described.
NIID is a highly heterogeneous disease and is characterized by various symptoms, including cognitive decline, parkinsonism, cerebellar ataxia, peripheral neuropathy, and autonomic dysfunction. Reference Fang, Yu and Yao3 The pathogenesis of NIID is still unclear. Immunohistochemically, the intranuclear inclusions are positive for ubiquitin and ubiquitin-related proteins, indicating that the nuclear ubiquitin-proteasome system may play an important role in NIID. Reference Tian, Wang and Huang4 The identification of GGC repeat expansion at the 5′ end of NOTCH2NLC as the genetic cause of NIID may help researchers identify the molecular pathogenesis of NIID. Reference Tian, Wang and Huang4
In 2011, Japanese scholar Sone diagnosed NIID based on skin biopsy. Reference Sone, Kitagawa and Sugawara5 In addition, characteristic high-intensity signals in U-fibers on brain MRI diffusion-weighted imaging (DWI) has become another indicator for NIID diagnosis. However, only a handful of studies have reported the clinical manifestations and neuroimaging features of sporadic NIID patients. There is an urgent need to better understand the clinical characteristics and radiological features of NIID. In this retrospective study, we studied the clinical, radiological, pathological, and electrophysiological features of 17 sporadic NIID patients in China.
Materials and Methods
Subjects
Patients with sporadic NIID were recruited from the database of our hospital between 2014 and 2021. Detailed clinical information for each patient was obtained from the patient, the patient’s family or clinical records. Information regarding age of onset, the progression of the disease, family history, and other clinical manifestations was collected. Detailed neurological examinations were performed by neurologists. The patients were diagnosed based on skin biopsy, and they underwent brain MRI, electrophysiological and cerebrospinal fluid (CSF) examinations.
This study was performed with ethical approval from the Ethics Committee of Ruijin Hospital affiliated with Shanghai Jiao Tong University School of Medicine. Written informed consent was obtained from all patients.
Brain MRI
All MRI examinations were performed on a standard clinical 3.0-T MRI scanner. Conventional T1-weighted spin-echo images (T1WIs), T2-weighted spin-echo images (T2WIs), fluid-attenuated inversion recovery (FLAIR) images, DWI images, and apparent diffusion coefficient images of the head were obtained according to our MRI protocol. All images were analyzed by an experienced radiologist (with 10 years of specialized experience) who was blinded to the clinical information.
Pathological Examinations of Skin Biopsy
Skin biopsy was performed in all 17 cases. Biopsy was typically performed 10 cm above the lateral malleolus after local anesthesia with 2% lidocaine following established procedures. The specimens were fixed in formalin solution, embedded in paraffin, cut into 6 μm-thick sections and stained with hematoxylin and eosin (H&E), an anti-ubiquitin antibody (Z0458, Dako), and an anti-p62 antibody (sc-28359, Santa Cruz Biotechnology) according to standard procedures. Reference Sone, Mori and Inagaki1
Statistical Analyses
Descriptive statistical analysis of frequencies and percentages for categorical variables and means and standard deviations for numerical variables was performed. For comparisons of continuous variables, t-tests were used. Statistical significance was defined as P < 0.05. All statistical analyses were performed using GraphPad Prism 8 and SPSS version 26.
Results
A total of 17 patients, including 8 males and 9 females, were enrolled (see Table 1). The average age of onset was 60.18 years, and the average age at diagnosis was 64.24 years. Tremor, the most common symptom, was observed in 11 (64.71%) patients, and 8 (47.06%) patients had tremor as the initial symptom. Cognitive impairment was the second most common symptom (10/17, 58.82%). Five (33.33%) patients developed encephalitic episodes. Peripheral neuropathy (6/17, 35.29%) and pyramidal symptoms (5/17, 29.41%) also appeared frequently and were followed in incidence by autonomic dysfunction (4/17, 23.53%), epileptic seizures (2/17, 11.76%), and extrapyramidal symptoms (1/17, 5.88%). One patient had NIID-related nephropathy before CNS symptoms appeared.
Table 1: Summary of the clinical features of the patients with NIID
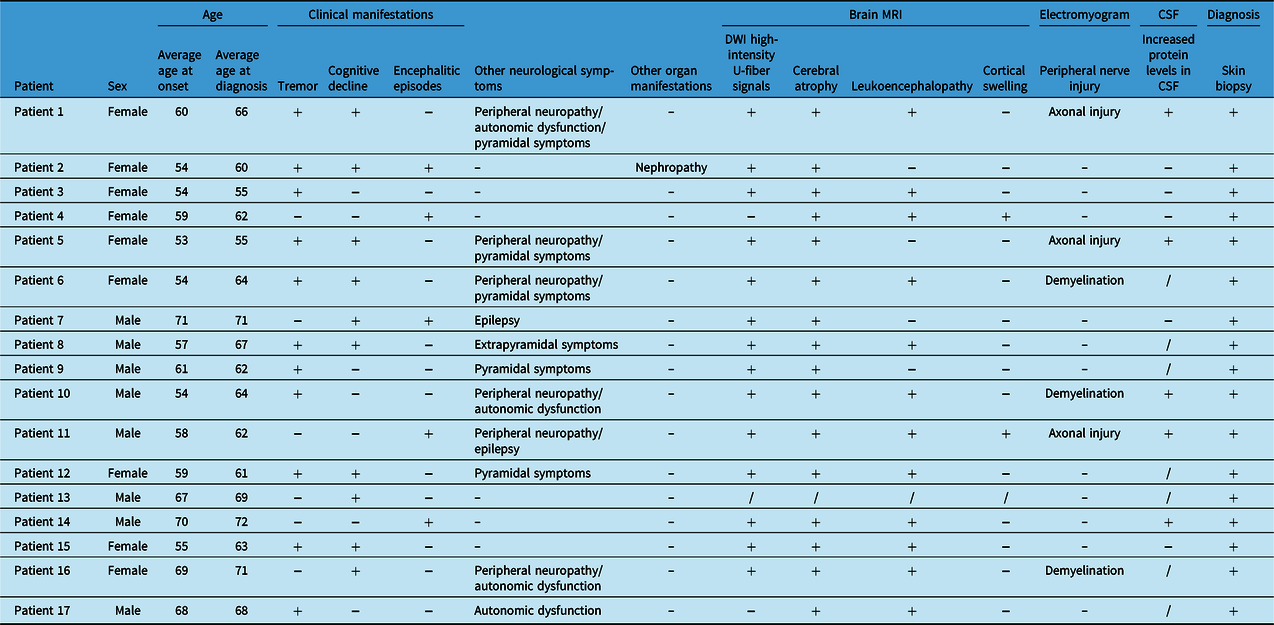
High-intensity U-fiber signals on DWI were observed in 14 cases, brain atrophy was found in 16 cases, leukoencephalopathy was seen in 12 cases and cortical swelling was observed in 2 cases. Other MRI findings included enlarged ventricles, cerebral sulci, and gyri. All patients underwent electrophysiological examination, and six patients presented peripheral neuropathy. Of these patients, three had axonal injury, and three had demyelination.
The skin biopsies of all 17 patients showed round P62-positive intranuclear inclusions in sweat glands, fibroblasts, and vascular endothelial cells (see Figure 1). All patients underwent genetic testing for FMR1 CGG repeat expansion to exclude fragile X-associated tremor/ataxia syndrome (FXTAS).
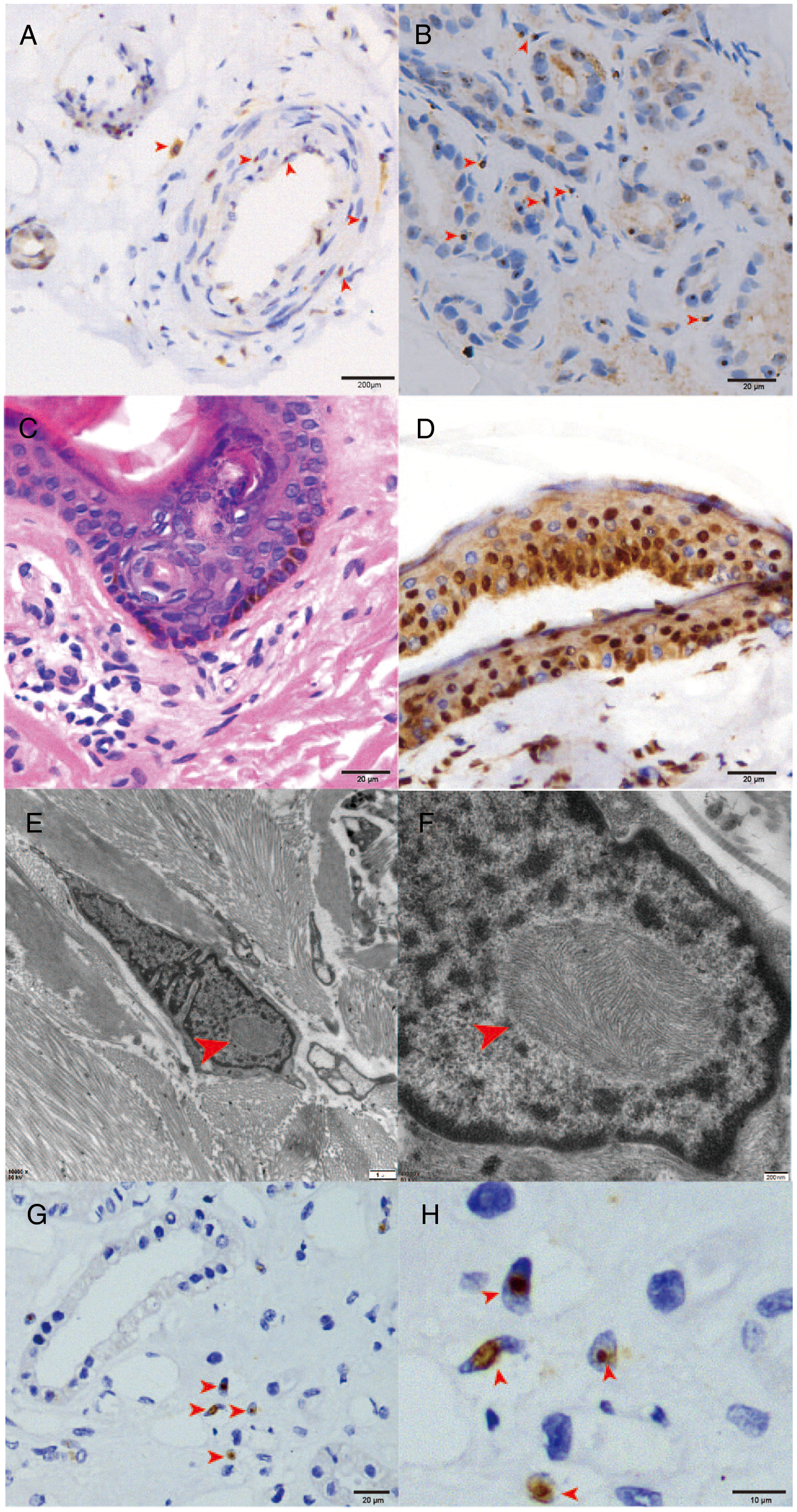
Figure 1: Biopsy histological and immunohistochemical findings. Light microscopy showed p62-positive intranuclear inclusions in vascular smooth muscle cells (A) and sweat gland cells (B). H&E-stained sections (C) and ubiquitin immunostaining sections (D) showed no obvious abnormality. Electron microscopy revealed inclusion bodies in fibroblasts (E and F). Renal biopsy also demonstrated p62-positive eosinophilic hyaline intranuclear inclusions in renal tubular cells (G and H).
Tremor
Tremor was seen in 11 cases and was the initial symptom in 8 patients, among which five had postural tremor, two had static tremor, and one had intentional tremor. The age at onset was lower in patients with tremor than in patients without tremor (57.18 ± 4.32 versus 65.67 ± 5.22, p = 0.0042) (Table 2).
Table 2: Comparison of age at onset and diagnosis between patients with and without tremor

* P < 0.05.
Encephalitic Episodes
Encephalitic episodes were another feature in our cohort. Patients that experienced encephalitic episodes presented with acute-onset fever, headache, and altered mental status. Cortical swelling and focal leukoencephalopathy were the imaging features in patients with encephalitic episodes. All patients with encephalitis received lumbar puncture, and CSF analysis showed normal pressure, leukocyte number, and glucose levels. However, protein levels were slightly elevated in two patients (2/5, 40%).
Multiple Organ Involvement
One NIID patient had a history of chronic renal insufficiency for more than 10 years. She was diagnosed with nephrotic syndrome and stage 4 renal failure. Skin biopsy showed p62-positive eosinophilic hyaline intranuclear inclusions in fibroblast cells, sweat gland cells, and adipose cells. Renal biopsy also revealed similar intranuclear inclusions in renal tubular cells (see Figure 1, E and F). Interestingly, her renal insufficiency preceded her CNS symptoms by 5 years.
Brain MRI Features
Brain MRI showed diffuse high-intensity signals of cerebral white matter on FLAIR images, and DWI showed high-intensity signals along the corticomedullary junction (see Figure 2, A). Cerebral atrophy and white matter degeneration were also observed. And we have found no imaging abnormalities in the cerebellum.

Figure 2: Brain MRI findings. (A) patient 3; (B–D) patient 11; (E–H) patient 4. Hyperintense linear lesions along the corticomedullary junction were observed on DWI images (A). From 2014 (B) to 2018 (D), the brain MRI of patient 11 progressively deteriorated, and extensive swelling of the right cerebral hemisphere and unclear sulci were observed on T2-FLAIR (C–D). Patient 4 had cortical swelling in the left temporal and occipital lobe on T2-FLAIR (F). There were no typical hyperintensity signals at the corticomedullary junction on DWI throughout the course of disease in patient 4 (E–H).
In patients with encephalitic episodes, we found some unique imaging findings. For example, patient 4 presented with recurrent episodes of encephalitis with reversible cortical swelling, worsening leukoencephalopathy, and brain atrophy. She was finally diagnosed with NIID based on skin pathology despite not having the typical sign of a high-intensity DWI signal along the corticomedullary junction (see Figure 2, E–H).
In addition, the abnormal high-intensity signal on DWI was dynamic in patient 11. During his clinical visit in 2014, he presented with recurrent fever and encephalitis for one month. Brain MRI showed bilateral periventricular leukoencephalopathy, and no obvious abnormalities were found on DWI. Four years later, when the patient returned for another visit due to the onset of encephalitis, DWI showed high-intensity signals in the bilateral frontal lobe and right temporal parietal occipital lobe, as well as aggravated white matter degeneration (see Figure 2, B–D).
Discussion
To the best of our knowledge, only few studies of adults-onset NIID have previously been discussed in the literature in China, and most studies are case reports. Reference Cao, Wu and Yue6–Reference Wang, Ma and Shi8 Herein, we summarized the clinical manifestations, pathological, and imaging features of 17 patients. NIID similarly affected patients of both sexes in our cohort. The average age of onset for symptoms was 60.18 years and the average time from onset of symptom to diagnosis was 4.06 years. Compared with the age of onset in the literature, our patients have symptoms at a relatively young age (60.18 versus 63.4). Reference Zhang, Wu, Zhu, Ni and Zhang9
Jia’s team found that dementia is the most common initial symptom of adult-onset NIID. Reference Wang, Ma and Shi8 Most patients with sporadic NIID present with dementia as the initial and main clinical manifestation. However, in our retrospective study, we found that tremor was the most common symptom. Patient 8 had progressive resting tremor in the right hand accompanied by bradykinesia. He was initially misdiagnosed with Parkinson’s disease. Kitagawa reported a 73-year-old woman with NIID who presented with slowly progressive resting tremor in both hands. Levodopa and trihexyphenidyl were ineffective against the tremor. Reference Kitagawa, Sone, Sobue, Kuroda and Sakurai10 O’Sullivan reported juvenile parkinsonism in a patient with NIID. The patient showed asymmetric arm tremor and bulbar symptoms. Widespread hyaline intranuclear inclusions and neuronal depletion in the substantia nigra were observed at autopsy. Reference O’Sullivan, Hanagasi, Daniel, Tidswell, Davies and Lees11 In addition, some of our patients had postural tremor and were initially misdiagnosed with essential tremor. Similarly, in our patients, drugs did not effectively treat tremors. We suggest that NIID should be suspected if tremor is severe and is poorly responsive to drug treatment. In our retrospective study, the age at onset of patients with tremor was lower than that of patients without tremor. Cognitive impairment occurred in 7 of the 11 patients with tremor, who presented with very mild cognitive decline.
In addition to tremor and cognitive impairment, the patients with NIID in our cohort had several other clinical manifestations. Li reported a patient with NIID who had multiple reversible encephalitic attacks and remained stable and nonprogressive for 7 years. Reference Li, Li and Li12 However, we found that five patients with multiple encephalitic episodes progressively deteriorated. Compared with patients without encephalitic episodes, patients with encephalitis were more likely to have fever, nausea and vomiting, confusion, and incontinence. The imaging feature of patients with encephalitic episodes was focal cortical swelling. Based on our long-term follow-up, encephalitic episodes followed by reversible asymmetric leukoencephalopathy, which were seen in our patients, may be a new indication for disease. Furthermore, cerebral atrophy may occur after encephalitic episodes. We speculate that each attack may aggravate cerebral atrophy. We suggest that NIID should be suspected in patients with encephalitic episodes followed by leukoencephalopathy. Reference Zhou, Luan, Chen and Liu13
Although NIID most commonly affects the nervous system and most pathological evidence has been observed in brain tissues, NIID occasionally involves multiple systems. Here, we present a case of NIID in which severe renal failure occurred previously. P62-positive eosinophilic hyaline intranuclear inclusions were found not only in fibroblast cells but also in renal tubular cells. Interestingly, the patient’s renal insufficiency preceded her CNS symptoms by 5 years. We speculated that this renal insufficiency was associated with NIID. Motoki reported that eosinophilic intranuclear inclusions can occur in renal tubular cells 12 years before diagnosis. Reference Motoki, Nakajima, Sato, Tada, Kakita and Arawaka14 Horino first reported a case of NIID associated with lupus nephritis-like nephropathy. Reference Horino, Matsumoto, Inoue, Ichii and Terada15 The patient was treated with steroids and angiotensin-converting enzyme inhibitors and his proteinuria improved. However, our patient eventually developed renal failure during long-term follow-up. Therefore, our case suggested NIID may cause severe renal insufficiency, and we need more cases to study the causal relationship between renal insufficiency and NIID.
The MRI features of our patients including high-intensity U-fiber signals on DWI, brain atrophy, leukoencephalopathy, cortical swelling and enlarged ventricles, cerebral sulci, and gyri. There are other specific MRI features not yet seen in our patients but reported in other literature including T2 hyperintensities in the bilateral paravermal regions and/or bilateral middle cerebellar peduncles. Reference Pang, Yang and Yuan16,Reference Chen, Lu and Wang17 Sone reported that high-intensity signals in the corticomedullary junction on DWI are highly characteristic of NIID. Reference Sone, Mori and Inagaki1 This abnormal hyperintense signal usually first appears in the frontal lobe and then develops in the parietal and occipital lobes. Reference Yokoi, Yasui and Hasegawa18 This continuous and linear high-intensity signal on DWI can also extend into the deep white matter. Reference Sugiyama, Sato and Kimura19 Interestingly, in our center, 14 patients showed high-intensity signals on DWI. One patient without high-intensity U-fiber signals on DWI had recurrent episodes of encephalitis, focal leukoencephalopathy, and progressive brain atrophy. We followed up with this patient for 4 years, and typical hyperintensity at the corticomedullary junction was still absent. Like the patient mentioned above, another patient at our center did not show a high-intensity signal on DWI at the first visit (patient 11, Figure 2, B–D). During long-term follow-up and after several encephalitic episodes, high-intensity signals on DWI appeared. To date, there have been few reports on the longitudinal course of hyperintensity on DWI. We suggest that although abnormal high-intensity DWI signals are indicative of NIID, negative findings do not exclude the diagnosis. We speculate that imaging examinations show dynamic changes as the disease progresses. Reference Raza, Singh and Rai20
In recent studies, the presence of repeat expansions in the 5’UTR of the NOTCH2NLC gene was shown to aid the diagnosis of NIID. One limitation of our study is that the CGG repeat length of the NOTCH2NLC gene was not analyzed. Skin biopsy and imaging results confirmed our diagnosis of NIID.
In summary, we analyzed the clinical characteristics of NIID patients who were diagnosed through skin biopsy and MRI. The prevalence of tremor in Chinese patients with NIID is likely to be higher than that reported in previous studies. Although MRI findings play an important role in the diagnosis of NIID, we should be aware that not all patients show the typical high-intensity signals on DWI in the corticomedullary junction at all times.
Acknowledgments
We would like to thank Xinghua Luan for performing and analyzing skin biopsies.
Funding
This study was funded by the development fund for Shanghai talents, Shanghai Shuguang Plan Project (18SG15), and Shanghai Young Top-notch Talent Support Program.
Conflicts of Interest
None.
Ethical Approval
This study was performed with ethical approval from the Ethics Committee of Ruijin Hospital affiliated with Shanghai Jiao Tong University School of Medicine. Written informed consent was obtained from all patients.
Statement of Authorship
Sheng Chen and Haiyan Zhou supervised the study. You Ni wrote most of the original draft. Zhao Yang guided the writing of the original draft. Zhao Yang performed and analyzed skin biopsies. Qinming Zhou helped to obtain information for the patients.
Jun Liu directed the original manuscript. All authors read and approved the final manuscript.
Data Availability Statement
All relevant data are described in the paper.






