Background and Purpose
Acute ischemic stroke affects 62,000 Canadians annually, Reference Benjamin, Virani and Callaway1 and is the third leading cause of death. 2 Several randomized control trials (RCTs) have demonstrated the superiority of endovascular thrombectomy (EVT) over standard medical care for acute ischemic stroke caused by intracranial anterior circulation large vessel occlusions (LVO). Reference Saver, Goyal and Bonafe3 – Reference Campbell, Mitchell and Kleinig8 EVT is now recommended as standard of care, and was integrated into the Canadian Best Practice Recommendations for Stroke Care in 2015. Reference Powers, Derdeyn and Biller9 , Reference Casaubon, Boulanger and Blacquiere10
Despite proven efficacy, there is limited evidence supporting EVT’s effectiveness. Effectiveness studies are necessary because health administrators prioritize local organizational data for systems-level decision-making. Reference Guo, Farnsworth and Hermanson11 Thrombectomy is technically complex and resource-intensive; hence, EVT-capable centers are concentrated in urban areas, which poses challenges to the equitable and timely delivery of EVT to patients living in rural and remote parts of Canada. Reference Fleet, Bussieres and Tounkara12
We studied the effectiveness of EVT in routine practice at a tertiary, academic center in Nova Scotia (population approximately 980,000). Using a matched case–control design, we assessed the benefit of EVT by comparing the intervention group with patients who were not treated with EVT but received the same routine medical treatments, including intravenous alteplase (tPA). We anticipate the results will inform the planning process to expand the delivery of EVT in our region.
Methods
Stroke patients treated with EVT from February 1, 2013 to January 31, 2017 were matched 1:1 with controls from February 1, 2009 to January 31, 2013 at the Queen Elizabeth II Health Sciences Centre (QEII) in Halifax, Nova Scotia, Canada. The QEII is an academic, urban, tertiary care, regional stroke center. It is the largest neurologic institute in Atlantic Canada, and the only EVT-capable hospital in the province of Nova Scotia. The study was approved by the institutional research ethics board.
Patients were identified from the QEII Acute Stroke Registry (ASR), maintained prospectively since 1997. Reference Phillips, Eskes and Gubitz13 Cases (n = 44) were patients who underwent EVT after our participation in the Endovascular treatment for Small Core and Anterior circulation Proximal occlusion with Emphasis on minimizing CT to recanalization times (ESCAPE) trial, Reference Goyal, Demchuk and Menon4 February 1, 2013 through January 31, 2017. Eligibility for EVT was determined using the ESCAPE trial criteria. Clinical inclusion criteria were patients older than 18 years (no upper-age limit) with a disabling ischemic stroke, who were functionally independent before the stroke and arrived at the QEII within 12 h of stroke onset (in-hospital strokes were excluded). Imaging criteria were as follows: LVO, defined as occlusions of the middle cerebral artery before the bifurcation (M1), two M2 branches (M1 equivalent), or distal internal carotid artery; small-core infarct, defined as an Alberta Stroke Program Early CT Score (ASPECTS) of 6–10 Reference Barber, Demchuk, Zhang and Buchan14 ; moderate-to-good collaterals on multiphase CT angiography Reference Menon, Qazi, Almekhlafi, Hahn, Demchuk and Goyal15 ; and small core with large penumbra seen on CT perfusion. Reference Jai, Shankar, Langlands, Doucette and Phillips16 , Reference Du and Shankar17
Controls were out-of-hospital stroke patients, who received standard medical therapy excluding EVT ± tPA. They were identified from the registry as having been admitted between February 1, 2009 and January 31, 2013 (prior to our center’s routine delivery of EVT), matched 1:1 to the cases in a hierarchical process based on age ± 5 years; prestroke functional status, quantified by the Oxford Handicap Score Reference Swieten, Koudstaal, Visser and Gijn18 (a variant of the modified Rankin Score [mRS]) and categorized as independent (0–2) or dependent (3–5); stroke syndrome (Oxfordshire Community Stroke Project [OCSP] classification Reference Bamford, Sandercock, Dennis, Burn and Warlow19 ); stroke severity, defined using a validated score, Reference Reid, Gubitz and Dai20 , 21 and categorized as mild (0–4), moderate (5–7), or severe (8–10); tPA administration; and gender. Thirty-one case–control pairs were matched on all six variables, five patients were matched to the first five variables, seven patients were matched to the first four variables, and one patient was age-matched only.
Measurements
Information concerning patient demographics, prestroke functional status, stroke syndrome and severity, risk factors, thrombolytic therapy, computed tomography/computed tomography angiogram (CT/CTA) findings, workflow and process times, course in hospital, length of stay, discharge disposition, and postdischarge follow-up (vital status and functional status [mRS]) was obtained from the ASR and by review of electronic hospital records and imaging files. Two patients were followed up by phone. EVT success was defined as a score of 2b or 3 on the Thrombolysis in Cerebral Infarction (mTICI) scale. Reference Zaidat, Yoo and Khatri22 Intracranial bleeding post-EVT was categorized using the Heidelberg criteria. Reference vom Kummer, Broderick and Campbell23 Symptomatic hemorrhage was defined as a hematoma occupying 30% or more of the infarcted tissue with overt clinical deterioration.
Statistical Analysis
Means (standard deviations), medians (interquartile ranges [IQRs]), and frequency counts were used to summarize baseline patient characteristics. We used Chi-square test, Fisher’s exact test, Student’s t-test, or Wilcoxon rank-sum test, as appropriate, to compare differences between cases and controls. Survival probabilities and the corresponding 95% confidence intervals were estimated using Kaplan–Meier analysis and compared using the log-rank test. SAS STAT 12.1 version 9.4 (SAS Institute, Cary, NC, USA) was used for all statistical analyses.
Results
Patient Characteristics
Adequate balance between cases and controls was achieved from the matching process, and was further ascertained by comparing demographic and clinical parameters between the two groups (Table 1). One exception is dyslipidemia, which was more prevalent among the EVT-treated patients (46% vs. 30%, p = 0.037). One control patient who could not be matched on stroke syndrome and severity had a mild stroke. More EVT-treated patients than controls received tPA (75% vs. 57%), but this was not statistically significant (p = 0.07). CTA was performed in all 44 EVT-treated patients and only in 12 (27%) of controls (p < 0.001); this difference was anticipated because angiographic information was not required for treatment decision-making pre-EVT. Most EVT-treated patients had an M1 occlusion (86%), whereas four (9%) had carotid T or L occlusion, and two (5%) had M1-MCA equivalent. Collaterals were good in 33 patients (77%), intermediate in 8 (19%), and poor in 2 (5%).
Table 1: Characteristics of EVT-treated cases and matched controls
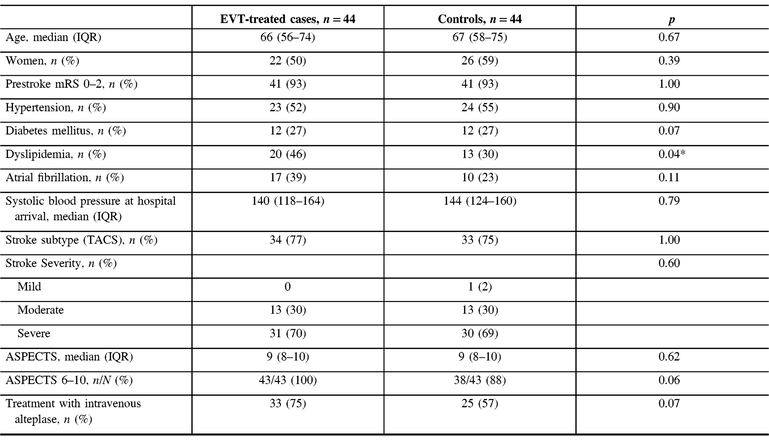
IQR = interquartile range; mRS = modified Rankin Score; TACS = Total Anterior Circulation Stroke syndrome of the Oxfordshire Community Stroke Project classification of ischemic stroke subtypes; ASPECTS = Alberta Stroke Program Early CT Score.
* p-values <0.05 are considered statistically significant.
Workflow and process times were captured. Three of the 44 EVT patients were transferred from a peripheral center for treatment. Stroke onset to first CT was a median of 107 min (IQR 70–227) for cases and 114 min (IQR 86–275) for controls. Median time to intravenous tPA administration was 139 min (IQR 107–170) in cases and 150 min (IQR 120–215) for controls. For EVT-treated patients, first reperfusion was obtained 247 min (IQR 200–367) after stroke onset, and successful recanalization (TICI 2b or 3) was obtained in 93%.
Outcomes
There was a mortality benefit favoring EVT-treated patients (Table 2). In-hospital mortality was 11% (n = 5) among the EVT-treated patients and 36% (n = 16) in the control group (p = 0.006). Of those who died in hospital, survival duration was not significantly different between the two groups with a median of 4 (IQR 0–4) days for cases versus 7 (IQR 0–11) days for controls (p = 0.17). The survival benefit conferred by EVT was sustained during long-term follow-up (Figure 1). One-year predicted survival was 85% for cases (standard error 0.15) and 57% (standard error 0.42) for controls (log-rank p = 0.01).
Table 2: Outcomes of EVT-treated patients and controls
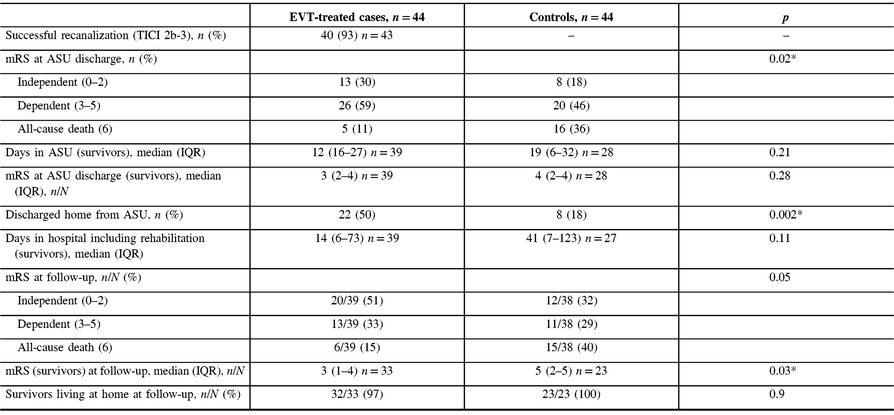
mRS = modified Rankin Score; IQR = interquartile range; ASU = Acute Stroke Unit; mTICI = modified Thrombolysis in Cerebral Ischemia score.
* p-values <0.05 are considered statistically significant.

Figure 1: Kaplan–Meier graph describing survival from index stroke. Endovascular thrombectomy-treated cases (dashed line) showed improved survival compared with controls that received standard medical treatment (solid line) (p = 0.014).
At discharge from the Acute Stroke Unit (ASU), EVT-treated patients had more favorable outcomes than controls when categorized as independent, dependent, or deceased (p = 0.02; Table 2). EVT-treated survivors spent less days on the ASU (12 days [IQR 6–27] vs. 19 days [IQR 6–32], p = 0.21), and were more likely to be discharged home (56% vs. 29%, p = 0.03). Thirteen of 39 (33%) EVT-treated patients and 15 of 28 (54%) controls (p = 0.1) were transferred for specialized in-patient rehabilitation where their median lengths of stay were similar (37 days [IQR 14–71] vs. 65 days [IQR 41–83], p = 0.11).
The median time to posthospitalization follow-up for survivors was 101 (IQR 84–169) days for cases and 146 (IQR 98–206) days for controls (p = 0.24). The distribution of mRS scores at follow-up is shown in Table 2 and Figure 2. Median mRS was better for surviving EVT patients than controls at ASU discharge and at follow-up. Similar proportions of survivors were living at home at follow-up.
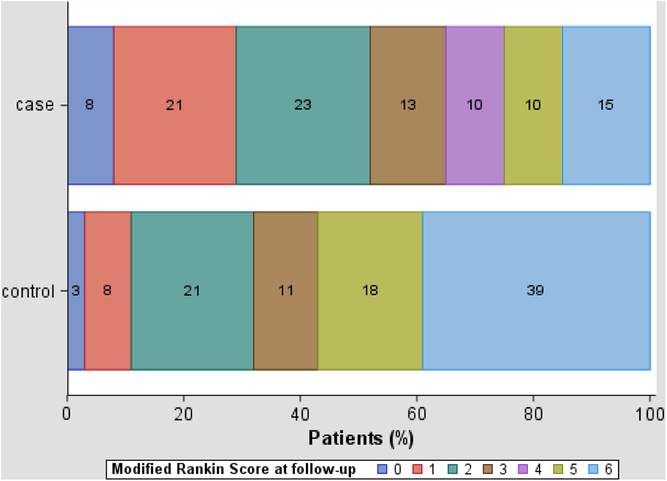
Figure 2: Modified Rankin Score at follow-up for EVT-treated cases (n = 39) and controls (n = 38) at 90-day follow-up.
Complications were infrequent. Forty-one (93%) of thrombectomy patients and 25 (57%) of control patients had a 24-h posttreatment CT head (p < 0.05). Hemorrhages were seen on the posttreatment scan of 12 (29%) EVT-treated patients, but only 4 had symptomatic clinical deterioration including 2 with parenchyma hemorrhage type 2. Of all hemorrhages seen, one was classified as a symptomatic SAH, five as PH1, and four as HI1 (petechiae).
Discussion
Our study supports the effectiveness of EVT. Using a case–control design, we offer evidence that EVT improves patients’ disability, mortality, and ability to return to independent living. EVT-treated patients had better outcomes than similar stroke patients who did not receive this therapy. This difference was particularly prominent early, at the time of discharge from the ASU. A greater proportion of EVT-treated patients survived their stroke, and were discharged home. This treatment benefit was still evident at follow-up. We inferred from our data that EVT-treated patients had a quicker, more meaningful recovery than controls. EVT-treated patients showed a consistent trend in favor of the intervention across all measures. More patients attained functional independence at discharge and on follow-up.
Notably, we showed a robust in-hospital survival benefit post-EVT, which was unique. ESCAPE was the only RCT to show a reduction in mortality, Reference Goyal, Demchuk and Menon4 whereas other trials were mortality-neutral. Reference Goyal, Menon and van Zwam24 Mortality among our EVT-treated patients was comparable to that reported in ESCAPE Reference Goyal, Demchuk and Menon4 and other RCTs Reference Goyal, Menon and van Zwam24 of EVT. Among the controls in our study, mortality was much higher than in the RCTs (36% vs. 19%), but in keeping with mortality rates for middle cerebral artery territory strokes observed in population-based studies. Reference Carandang, Seshadri and Beiser25,Reference Luengo-Fernandez, Paul and Gray26 Higher mortality in epidemiologic studies are attributed to heterogeneous patient populations, including those with prestroke comorbidity and disabilities, and failed recanalization after LVO strokes. This reflects patients’ and families’ wishes to withdraw active medical treatment in light of a poor prognosis. Reference Kelly, Hoskins and Holloway27 Our outcomes reflect routine treatment paradigms for severe strokes and strongly support intervention with EVT.
Although there was a consistent and strong trend favoring intervention seen across all outcome domains, particularly at discharge from ASU, we were unable to demonstrate statistically significant benefit across all outcome parameters at follow-up for several reasons. First, this is a single-center study with a small sample size, capturing our experience with EVT over 4 years. Second, we were unable to ascertain standardized 90-day follow-up mRS due to variations in clinical follow-up duration. Median time to follow-up for the control group was 45 days longer which may extend the window for neurologic recovery and disadvantage the treatment group in our functional analysis and comparison. Lastly, we included exceptional cases that did not fulfill trial criteria; for example, 2/44 (5%) cases had poor collaterals and 3/44 (7%) of patients were functionally dependent prestroke, which may have diluted the effect of EVT.
Other single-center retrospective studies in Canada, Europe, and Asia corroborate our results. They show high reperfusion rates and improved mRS at 3 months were reproducible in routine clinical practice, Reference Carvalho, Cunha and Rodrigues28 – Reference Wee, McAuliffe and Phatouros33 though in-hospital or 90-day mortality rates (20%–27.5%) in these studies were higher than the RCTs. They show that the advantage of EVT is generalizable across different health-care models. Comparatively, we achieved a relatively high-end procedure reperfusion rate of 91% (recanalization rates ranged from 68–93% in other single-center studies). Interventionists’ experience and proficiency, and second generation stent-retriever devices have led to our improved procedural outcome; exclusion of in-hospital stroke patients reduced the burden of comorbidities in our study population.
Evidence provided by our study supports the expansion of EVT in our province. Studies have shown that despite clinicians’ emphasis on evidence-based medicine, only 10% of health administrators use an evidence-based approach for greater than 75% of their decision-making Reference Guo, Hermanson, Farnsworth and Dean34 ; rather, they heavily rely on organizational data and personal experience. Using our study, we have generated a local cost–benefit analysis to stimulate the development of a more comprehensive stroke-network that will improve EVT accessibility for all patients, urban and rural. This is crucial, considering the expected growth in demand. New RCTs suggest that in a subset of highly selected patients, benefits of EVT may be seen up to 24 h after stroke onset. Reference Nogueira, Jadhav and Haussen35 , Reference Albers, Marks and Kemp36 At our center, thrombectomy rates have tripled since 2014. Studies suggest in the upcoming years, 10%–17% of all acute ischemic stroke patients will meet eligibility criteria for EVT, Reference Mcmeekin, White, James, Price, Flynn and Ford37 , Reference Vanacker, Lambrou, Eskandari, Mosimann, Maghraoui and Michel38 thus proactive development of a comprehensive EVT program provincially is essential.
Our study has several limitations. First, the matched case–control design has not controlled for all confounders. Demographics were comparable, though six patients were incompletely matched based on our preselected parameters. In addition, controls were identified over a wider time frame than the cases; however, our approach to evidence-based stroke care has remained consistent over two decades, during which time in-hospital mortality on our ASU has remained constant. We deliberately chose our controls prior to January 2013 (before we started enrollment for the ESCAPE trial) because patients with LVOs who did not receive EVT after 2013 at our center would have been unsuitable EVT candidates, perhaps due to their high burden of comorbid illnesses. Including controls from the same period as the cases (2015 and onward) would have introduced a bias in favor of EVT because untreated patients would likely have had worse-than-typical outcomes, which would have biased the results in favor of EVT. Lastly, retrospective review of clinical and radiologic information could have introduced reporting bias. However, many of our key outcome measures, including mRS at ASU discharge and discharge disposition, were obtained prospectively from our stroke registry. Outcomes such as mortality, length of stay, and discharge disposition are not subject to reviewer bias.
Conclusion
EVT at our center was effective compared with routine medical therapy alone. EVT reduced mortality, and it also showed superiority over standard medical therapy alone for improving functional outcome at discharge from hospital and at 3-month follow-up. This study highlights the importance of allocating adequate resources to sustain and expand the delivery of EVT.
Disclosures
None.
Statement of Authorship
SXH and KV (postgraduate trainees) co-led the case–control study and collected the patient data; WLS collects the data for the Acute Stroke Registry; CAC maintains the Acute Stroke Registry; HC devised the algorithm for matching the cases and controls; KJM provided expertise in statistical analysis; JJSS supervised the neuroradiological aspects of the study; SJP conceived the study and supervised the neurological aspects; all authors reviewed the manuscript.






