Introduction
Brain-derived neurotrophic factor (BDNF), a member of the neurotrophin family of survival-promoting molecules, plays an important role in the growth, development, maintenance, and function of several neuronal systems.Reference Hyman, Hofer and Barde 1 It is known to modulate synaptic plasticity and neurotransmitter release in a variety of neurotransmitter systems, as well as intracellular signal-transduction pathways.Reference Hyman, Hofer and Barde 1 It regulates axonal and dendritic branching and remodeling,Reference McAllister 2 - Reference Yacoubian and Lo 5 synaptogenesis in arborizing axon terminals, efficacy of synaptic transmission, and the functional maturation of excitatory and inhibitory synapses.Reference Rutherford, Nelson and Turrigiano 6 - Reference Vicario-Abejon, Collin, McKay and Segal 8
Brain-derived neurotrophic factor is believed to play a critical role in neuroprotection after brain insults and represents a crucial signaling molecule in adaptive brain plasticity after stroke. Several studies support increased BDNF production after stroke, as suggested by increased BDNF levels collected in areas, either at the lesion site or around the lesion for at least 1 week following stroke.Reference Comelli, Seren and Guidolin 9 , Reference Kokaia, Andsberg, Yan and Lindvall 10 - Reference Sulejczak, Ziemlinska, Czarkowska-Bauch, Nosecka, Strzalkowski and Skup 13 Moreover, after stroke, interventions that improve recovery of function are most often associated with increased BDNF levels in perilesional areas.Reference Chen, Zhang and Jiang 14 Yanamoto et al.Reference Yanamoto, Nagata and Sakata 15 demonstrated that direct intracerebral administration of exogenous recombinant-BDNF was shown to induce resistance against temporary focal ischemia in the rat neocortex. The molecular mechanisms for the neuroprotective property of BDNF under ischemic conditions are not fully understood: it can maintain calcium homeostasis of neurons,Reference Nakao, Kokaia, Odin and Lindvall 16 inhibit free radical production,Reference Petersen, Larsen and Behr 17 protect neurons against metabolic and excitotoxic insults,Reference Shimohama, Tamura and Akaike 18 and prevent neurons from apoptosis.Reference Schabitz, Sommer, Zoder, Kiessling, Schwaninger and Schwab 19
Post-stroke depression (PSD) is one of the most common and well-studied phenomena in post-stroke patients. Post-stroke depression affects up to 60% of all patients and is associated with increased morbidity and mortality following ischemic stroke.Reference Astrom, Adolfsson and Asplund 20 - Reference Robinson, Bolduc and Price 22 However, little is known about changes in BDNF in PSD. Growing evidence suggests important roles for reduced BDNF brain levels in the pathogenesis of mood disorders and in the mechanism of action of therapeutic agents, such as mood stabilizers and antidepressants.Reference Duman 23 - Reference Manji, Drevets and Charney 25
The aim of this study was to test for an association between brain BDNF protein levels and the development of depression following ischemic stroke. We used a middle cerebral artery occlusion (MCAO) rodent stroke model, as an animal model for PSD, whereas the two-way shuttle avoidance task, the Porsolt forced-swim test, and the sucrose preference test were all employed 30 days post-surgery to assess any depression-like behavior.
Experimental Procedures
All procedures were carried out under strict compliance with ethical principles and guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All treatment and testing procedures were approved by the Animal Care Committee of Ben-Gurion University of the Negev, Israel.
Animals
Adult male Sprague-Dawley rats (Harlan Laboratories Jerusalem, Israel) weighing 150-200 g were used (N=43). The animals were housed, four per cage, in a vivarium with stable temperature and a reversed 12-hour light/dark cycle (lights off at 08:00 a.m.), with unlimited access to food and water. All efforts were made to minimize the number of animals used and their suffering.
Experimental Design
Rats were randomly assigned to two groups: (1) MCAO group was subjected to a permanent MCAO surgery (N=24); and (2) sham group was used as a control sham-operated group without MCAO and was only subjected to a skin incision and anesthesia (N=19). In total, 50 minutes, 24 hours, and 7 days following the procedure, a neurological severity assessment was performed. Within each group, five rats were sacrificed after 24 hours to determine the size of the infarcted brain volume and brain edema, and the remaining rats were used for behavioral tests. Behavioral tests were carried out 30 days post-surgery. The rats were sacrificed 24 hours after the last behavioral test and their brains collected for the evaluation of BDNF protein levels (Figure 1).
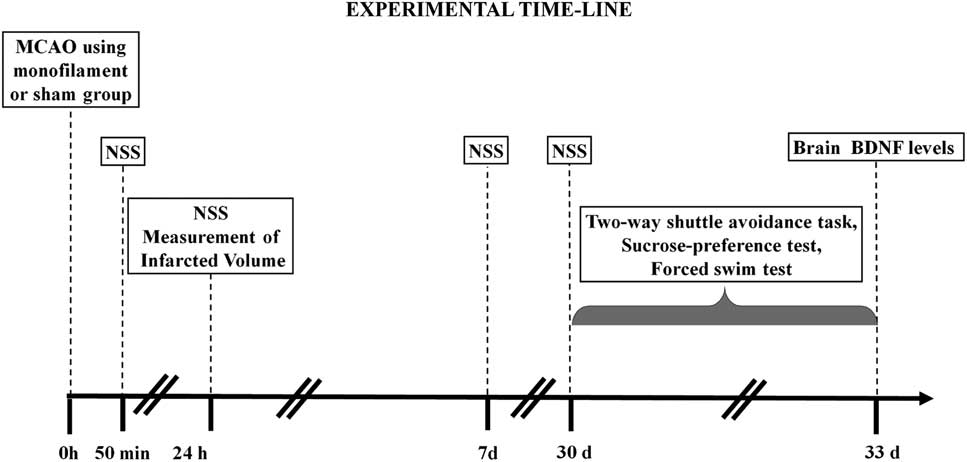
Figure 1 Experimental design: a schematic illustration of the experimental procedures. Rats were subjected to the different tests, at the different times indicated on the scheme: middle cerebral artery occlusion (MCAO) was performed at the start of the experiment; neurological severity score (NSS) was evaluated at 50 minutes, 24 hour, and 7 and 30 days after MCAO; The two-way avoidance, sucrose preference, and forced swim tests were conducted 30 days after MCAO; and brain-derived neurotrophic factor (BDNF) levels were measured 33 days after MCAO.
Surgical Procedures
The rats were anesthetized with a mixture of isoflurane (4% for induction, 2% for surgery, 1.3% for maintenance) in 24% oxygen (2 liters per minute), without tracheotomy, and allowed to breathe spontaneously. Rats were anesthetized for 45 minutes during each procedure. There were no differences in the time allotted for anesthesia between groups in order to control the effects of isoflurane, pO2, or pCO2. The anesthesia was considered sufficient for surgery when the tail reflex was abolished. Physiological parameters, including mean arterial pressure, heart rate, and O2 saturation of arterial blood, were monitored (Model: Horizon-XL; Mennen Medical Ltd, Yavne, Rehovot, Israel). A heating plate was used to maintain a core body temperature of 37°C, measured via a probe placed in the rats’ rectum. Body temperature was kept constant between rats, thus, minimizing any effect of hypothermia or hyperthermia on neurological outcome and neurological injury. The body temperature before surgery was measured from 8:00 to 9:00 a.m.in all animals and 24 hours after the onset of MCAO.
Surgical Procedures for Middle Cerebral Artery Occlusion
The surgery was performed according to a method highlighted in the procedure of Boyko et al.Reference Boyko, Zlotnik and Gruenbaum 26 In the original technique,Reference Longa, Weinstein, Carlson and Cummins 27 access to the middle cerebral artery (MCA) occurs via the external carotid artery (ECA). The occipital artery, ECA and its branches, terminal lingual and maxillary artery, are dissected distally and coagulated. The disruption of the ECA and its branches leads to impaired mastication because the vascular supply to the muscles of mastication is compromised.Reference Dittmar, Spruss, Schuierer and Horn 28 The muscles’ destruction caused by this method can lead to additional computational fluid dynamics release. In our new modified technique for stroke with MCAO occlusion via internal carotid artery (ICA), there is no disruption of blood flow in the ECA and its tributaries. Our technique offers better results including lower variability of infarct volume, better weight gain after stroke, and less mortality.Reference Boyko, Zlotnik and Gruenbaum 26 The MCA was occluded by inserting the monofilament directly through the ICA. Following a careful dissection and exposure of the common carotid artery (CCA) and ICA, and after separation of these arteries from the vagus nerve, the ICA was permanently blocked (a 4-0 silk suture was tied loosely around ICA just above the CCA bifurcation) proximal to the filament insertion point and temporarily blocked distal to the filament insertion point. The purpose of the proximal ligation was to occlude the ICA while the additional distal ligation reduced the bleeding around the filament and secured it in place. The heat-blunted monofilament was introduced via the ICA into the circle of Willis, effectively occluding the MCA. The silk suture in the ICA was fastened around the intraluminal thread to prevent bleeding. The suture was inserted ~18.5-19.0 mm from the bifurcation of the CCA until a sensation of mild resistance was reached to occlude the MCA. The filament was then fixed in position by tying a silk suture over the ICA, just above the pterygopalatine artery bifurcation. The duration of the surgery was 25-30 minutes.
The sham-operated group rats were operated in the same way as the experimental group rats, except for the insertion of the nylon thread.
Neurological Severity Score (NSS)
An observer, who was blinded to the surgical procedure, tested the animals for neurological deficits following MCAO, using methods which grade motor deficits on a cumulative scale from 0 to 4.Reference Boyko, Ohayon and Goldsmith 29 According to this scale, a score of 0 was given for no visible neurological deficits; a score of 1 was given for forelimb flexion; a score of 2 was given for contralateral weak forelimb grip (the operator places the animal on an absorbent pad and gently pulls the tail); a score of 3 was given for circling to the paretic side only when pulled by the tail (the animal was allowed to move about freely on the absorbent pad); and a score of 4 was given for spontaneous circling. Neurological severity score evaluation was performed at 50 minutes, 24 hours, 7, and 30 days following the procedure.
Measurement of Infarcted Volume
Infarcted volume was determined using the 2,3,5-triphenyltetrazolium chloride (TTC) staining as previously described.Reference Boyko, Ohayon and Goldsmith 30 The rats were euthanized by replacing their inspired gas mixture with 20% O2/80% CO2 and decapitated. Their brains were quickly isolated and sliced coronally into serial 2-mm-thick slices. The set of slices from each brain was incubated for 30 minutes at 37°C in 0.05% TTC. Following staining, the slices were scanned with an optical scanner (Canon CanoScan 4200F, Surrey, UK; resolution 1600×1600 dpi). The area of the brain edema was expressed as a percentage of the normal areas in the contralateral unaffected hemisphere. The total size of infarction was obtained by numeric integration of the area of marked pallor measured in six consecutive 2-mm coronal sections. In order to correct for tissue swelling, the following formula was applied: Corrected infarct size=infarct size×contralateral hemisphere size/ipsilateral hemisphere size. We measured the infarcted brain volume as a percentage of the total brain, striatum, and cortex.Reference Ohayon, Boyko and Saad 31
Behavioral Paradigms
The behavioral paradigms were chosen to cover a range of unconditioned and conditioned models of depression, and were performed 30 days post-surgery. All behavioral tests were performed in a closed, quiet, light-controlled room in the Faculty of Medicine, Anxiety and Stress Research Unit, Ben-Gurion University between 09:00 a.m. and 17:00 p.m. All behavioral tests were video-recorded for future analysis using the ETHO-VISION program (Noldus), by an investigator blinded to the experimental protocol. Rats underwent more than one behavioral test, and therefore, tests were performed with a break of at least 24 hours between sessions. Also, no subjects underwent the same test twice.
Exploratory Behavior in a Novel Setting
Exploratory behavior in a novel setting was assessed in the two-way shuttle avoidance apparatus, while it was in an inoperative state. Rats were first placed in the apparatus, described below, before it was turned on, and were allowed to explore both compartments for 10 minutes. If a rat did not shuttle over to the adjacent compartment after 5 minutes, it was manually and gently directed through the passage door. The same was done if the rat failed to shuttle back 5 minutes later. An observing experimenter recorded the rats’ back and forth exploratory shuttles between compartments. Only voluntary exploratory shuttles were counted.
The Two-Way Shuttle Avoidance Task
Immediately following the exploratory behavior assessment, rats were trained in the two-way shuttle avoidance task in a single 100 trials session.
Apparatus
The two-way shuttle avoidance box, placed in a dimly lit, ventilated, sound-attenuated cupboard, is a rectangular chamber (60×26×28 cm) divided by an opaque partition with a small (10×8 cm) passage connecting two equal sized side by side cube-shaped compartments. Both compartments’ metal grid floors are weight sensitive, micro-switches that transmit information about the rat’s location to a computer data collection control and program managing both conditional stimulus (CS) presentations (a tone produced by speakers located on the compartments’ distal walls) and electric shocks deliveries (Gimini; San-Diego Instruments, San Diego, CA).
Procedure
One session comprised of 100 “trace conditioning” trials. CS: 10-second tone presentation; unconditional stimulus (US): immediately following the termination of the CS an electric shock (0.5 mA) was delivered for a maximum of 10 seconds; I.T.I.: (randomly varying) 60±12 seconds.
Rats could produce one of three responses: (1) avoidance: shuttling to the adjacent compartment upon hearing the CS-tone; the tone stopped and an intertrial interval (I.T.I.) started; the rat avoided the electric shock. (2) Escape: shuttling to the adjacent compartment after the US-shock started; the shock stopped and an I.T.I. started. (3) Escape-failure: failing to move to the adjacent compartment; the I.T.I. commenced at the end of the 10-second foot shock. Thus, the rat was subjected to the full duration of the electric shock.Reference Boyko, Kutz and Gruenbaum 32
Sucrose Preference Test
The sucrose preference test is a measure of the “hedonic” state of an animal, or the ability to experience pleasure. Its impairment is a fundamental feature of clinical depression.Reference Association 33 , Reference Boyko, Kutza and Grinshpun 34
Procedure
In all experiments, before the first sucrose preference test, all the rats were subjected to 48 hours of forced exposure to 1% sucrose solution in order to habituate to it, during which sucrose solution was the only fluid available for consumption, followed by 2 days of free access to food and water.
The sucrose preference test was performed in the rat’s home cage. Rats were offered a free choice for 24 hours between two bottles, one containing 1.0% sucrose solution and the other, tap water. To prevent possible effects of side preference in drinking, the position of the bottles was switched after 12 hours. The animals were not deprived of water or food before the test. The consumption of water and sucrose solution was measured by weighing the bottles. The preference for sucrose was calculated from the amount of sucrose solution consumed, expressed as a percentage of the total amount of liquid drunk, according to the following ratio:
Porsolt forced-swim test
The test is based on the observation that rats, when forced to swim in a restricted space from which they cannot escape, will eventually cease apparent attempts to escape and become immobile, apart from the small movements necessary to keep their heads above water. Porsolt et al.Reference Porsolt, Anton, Blavet and Jalfre 35 , Reference Boyko, Azabb and Kutsa 36 suggested that this characteristic and readily identifiable behavioral immobility reflect a state of despair in the rat and showed that immobility was reduced by a variety of agents which are therapeutically effective in depression.
Procedure
Rats were individually forced to swim inside vertical plexiglass cylinders (height: 40 cm; diameter: 18 cm) containing 15 cm of water maintained at 25°C. After 15 minutes in the water, they were removed and allowed to dry for 15 minutes in a heated enclosure (32°C) before being returned to their home cages. They were replaced in the cylinder 24 hours later and the total duration of immobility was measured during a 5-minute test. Each rat was judged to be immobile whenever it remained floating passively in the water in a slightly hunched but upright position, its head just above the surface.Reference Boyko, Azabb and Kutsa 36 , Reference Porsolt, Le Pichon and Jalfre 37
Brains
Brains were immediately removed from the decapitated rats. Dentate gyrus (DG), Cornu Ammonis 1 (CA1) and CA3 sub-regions of the hippocampus were dissected according to Paxinos and WatsonReference Paxinos and Watson 38 and frozen at −70°C until used.
Western blot analysis
Brain samples extracted from rats’ CA1 and DG were sonicated for 15 seconds at 4°C and at 50% power capacity (Heat System Ultasonic Inc.) in 300 μl lysis buffer. After centrifugation at 10,000 g, for 15 minutes, at 4°C, the supernatant was transferred into new tubes and the protein concentration determined (Bradford, Bio-Rad). Samples were stored at −20°C until assayed. Protein samples (2 μg) were separated on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, blotted for 2 hours on polyvinylidene difluoride membrane and blocked with tris-buffered saline/TWEEN 20 (TBS/T) (0.61% Tris/HCl, 0.59% NaCl, 1% Tween 20, pH 7.7) containing commercial 5% non-fat dry milk and incubated overnight at 4°C with BDNF-specific antibody (1:1000; Santa-Cruz Biotechnology, CA). The membranes were washed with TBS/T and incubated for 1 hour with an a horseradish peroxidase-secondary antibody. The specific bends were detected with ECL plus kit (Amersham Bioscience, Piscataway, NJ) and a sensitive film (Kodak). Quantification was performed by El Logic 100 imaging system (Kodak) and molecular weight analysis software (Kodak). To minimize the effect of inter-blot variability, all samples were run in duplicates on the same gel and densitometric comparisons were made between control and treated samples from the same gel.Reference Boyko, Azabb and Kutsa 36
Unsupervised fuzzy clustering analysis
Clustering is a tool that attempts to assess the relationships among patterns in a data set by organizing the patterns into groups or clusters, such that patterns within one cluster are more similar to each other than to patterns belonging to other clusters.Reference Berg, Palomäki, Lehtihalmes, Lönnqvist and Kaste 39 - Reference Rezaee, Lelieveldt and Reiber 43 The main goal of most clustering methods is to supply useful information by grouping data in clusters, where within each cluster, the data exhibit similarity. Similarity is defined by a distance measure, whereas global objective, functional, or regional graph theoretic criteria are optimized to find the optimal partition of data. The partition approach defines to which cluster each data element belongs by using the elements of the membership matrix.Reference Berg, Palomäki, Lehtihalmes, Lönnqvist and Kaste 39 - Reference Rezaee, Lelieveldt and Reiber 43
Prior knowledge of the data set’s parameters (number of clusters, cluster means, membership matrix) or lack of such knowledge is usually a crucial factor in choosing the clustering method; when there is no membership matrix associated with the data set, the data must be termed unlabeled and the clustering method is thus unsupervised. In this case, there are several topicsReference Berg, Palomäki, Lehtihalmes, Lönnqvist and Kaste 39 referring to the data set and the clustering methods that need clarification:
1. The data structure used to determine the capability of cluster validity:
Different mathematical properties can be used to define the data structure; our choice was to examine the cluster validity in the c-normally distributed (Gaussian) groups because Gaussian data structures describe many natural signals and are well-defined mathematically.
2. The methods of classifying the data set into clusters:
Given that we chose to investigate Gaussian data, we used the unsupervised optimal fuzzy clustering algorithm,Reference Gath and Geva 42 which is accepted to represent a reliable method for clustering Gaussian data sets.
3. The methods for finding the actual number of clusters:
One of the main problems in unsupervised fuzzy clustering (UFC) is the estimation of the number of clusters in the data—the so-called cluster validity. Cluster validity is a difficult problem whose resolution is crucial for the practical application of clustering. In this study, the criteria applied are based on the fuzzy number of points in each cluster and the hypervolume of each cluster (the determinant of the cluster), which, after dividing the former by the latter, results in the cluster density, The main criteria which were used in this study are:
a. the number of clusters divided by the fuzzy version of the least square function;
b. one divided by the sum of the hyper-volumes of all clusters;
c. the sum of the fuzzy number of points in all clusters divided by the sum of hyper-volumes which construct the partition density; and
d. the mean of each cluster density which constructs the partition mean density.
For each number of clusters, a different partition of the data set was found (different membership matrix and means for the kth cluster). Once found, a validity criterion was applied to each partition in order to define the validation value. The optimal number of data classes can be determined based on a maximum of these validation values for all partitions. A full description of the UFC algorithm is presented in Cohen et al.Reference Cohen, Zohar, Matar, Kaplan and Geva 44
Statistical Analyses
Physiological variables, lesion volumes, and ratios of hemispheric diameters were compared with the use of the Mann-Whitney U test. The NSS at each time point was expressed as the median±(25-75th percentile) and were compared with the use of the Mann-Whitney U test. Behavioral and molecular data were analyzed using the one-way analysis of variance (ANOVA) and the post hoc Bonferroni tests for multiple comparisons. The prevalence of affected rats as a function of rat group was tested using cross-tabulation and nonparametric χ2 tests.
Results
Histology
There was a statistically, significant, larger infarcted volume as a percentage of total brain (8.8%±6.5 vs. 0.3±0.1%, p<0.0001), striatum (4.9%±2.3 vs. 0.2%±0.2, p<0.002), and cortex (3.9%±4.5 vs. 0.1%±0.1, p<0.00003) in the MCAO group when compared with the sham-control group. The MCAO group also had a statistically, significant, larger extent of brain edema when compared with the sham-control group (10.2±4.6% vs. 2.6±1.2%, p<0.0003).
Neurological Outcome
The NSS obtained 50 minutes, 24 hours, and 7 days in groups are presented in Table 1.
Table 1 Neurological severity score (NSS) in the middle cerebral artery occlusion (MCAO) and sham-operated groups
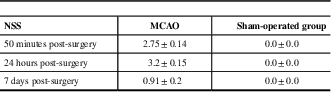
All data represent group mean±SEM.
The NSS scores were statistically different, demonstrating lower neurological performance in the MCAO group compared with the sham group (Mann-Whitney tests: 50 minutes: p<0.001, 24 hours: p<0.05, and 7 days: p<0.05).
Exploration of a Novel Setting, 30 Days After Middle Cerebral Artery Occlusion
The results showed that MCAO surgery significantly reduced exploratory behavior before the stressful challenge (F(1,28)=93.0, p<0.0001) (Figure 2A). Compared with sham, MCAO animals exhibited significantly less exploratory shuttles in a novel setting, namely the shuttle box, before avoidance learning (p<0.0001).
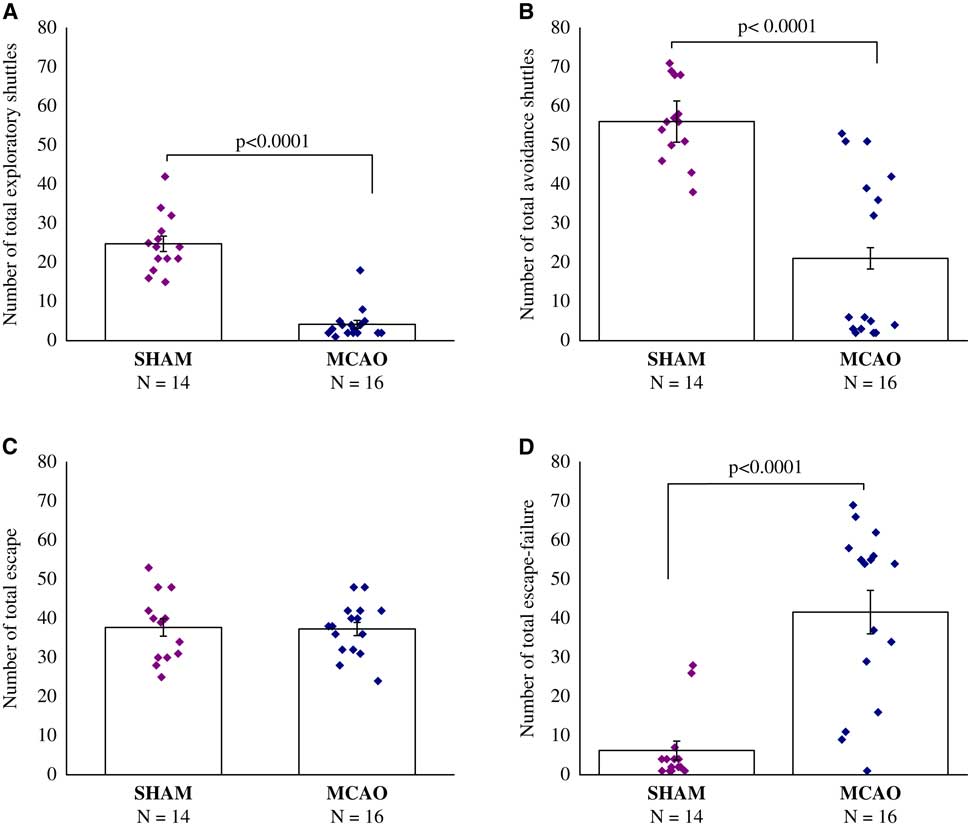
Figure 2 Two-way shuttle avoidance learning test, 30 days after middle cerebral artery occlusion (MCAO) (n=16) or sham (n=14). (A) Number of exploratory shuttles. (B) Number of total avoidance shuttles. (C) Number of total escape. (D) Number of total escape-failure. One-way analysis of variance revealed that middle cerebral artery occlusion surgery significantly reduced exploratory behavior before the stressful challenge (p<0.0001), and also developed a remarkable learned helplessness compared with the sham-operated. All data represent group mean±SEM.
Two-Way Shuttle Avoidance Learning, 30 Days After Middle Cerebral Artery Occlusion
The results showed, that rats in the MCAO group developed remarkably learned helplessness compared with the sham-operated. Compared with sham-operated rats, MCAO rats performed significantly fewer avoidance shuttles (F(1,28)=32.0, p<0.0001) (Figure 2B), and presented substantially higher rates of escape failures (F(1,28)=30.9, p<0.0001) (Figure 2D), while learning the two-way shuttle avoidance task. No differences were evident between sham-operated rats and MCAO rats in the number of total escape responses (Figure 2C).
Sucrose Preference Test, 30 Days After Middle Cerebral Artery Occlusion
We found a significant difference in sucrose consumption between sham-operated rats and MCAO rats, with MCAO rats consuming less sucrose (F(1,28)=12.5, p<0.0015) (Figure 3A).
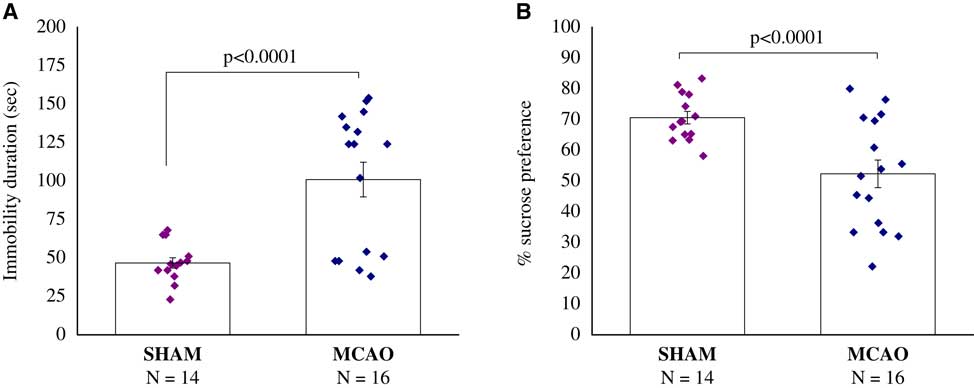
Figure 3 Sucrose preference test, 30 days after middle cerebral artery occlusion (MCAO) (n=16) or sham (n=14). (A) Immobility duration (second). (B) Percentage of sucrose preference. A significant difference in sucrose consumption between MCAO and sham-operated rats, with MCAO rats consuming less sucrose (p<0.0001). All data represent group mean±SEM.
Porsolt Forced-Swim Test, 30 Days After Middle Cerebral Artery Occlusion
The immobility time in the forced-swimming test was significantly longer in the MCAO group compared with the sham group (F(1,28)=18.9, p<0.0002) (Figure 4B).
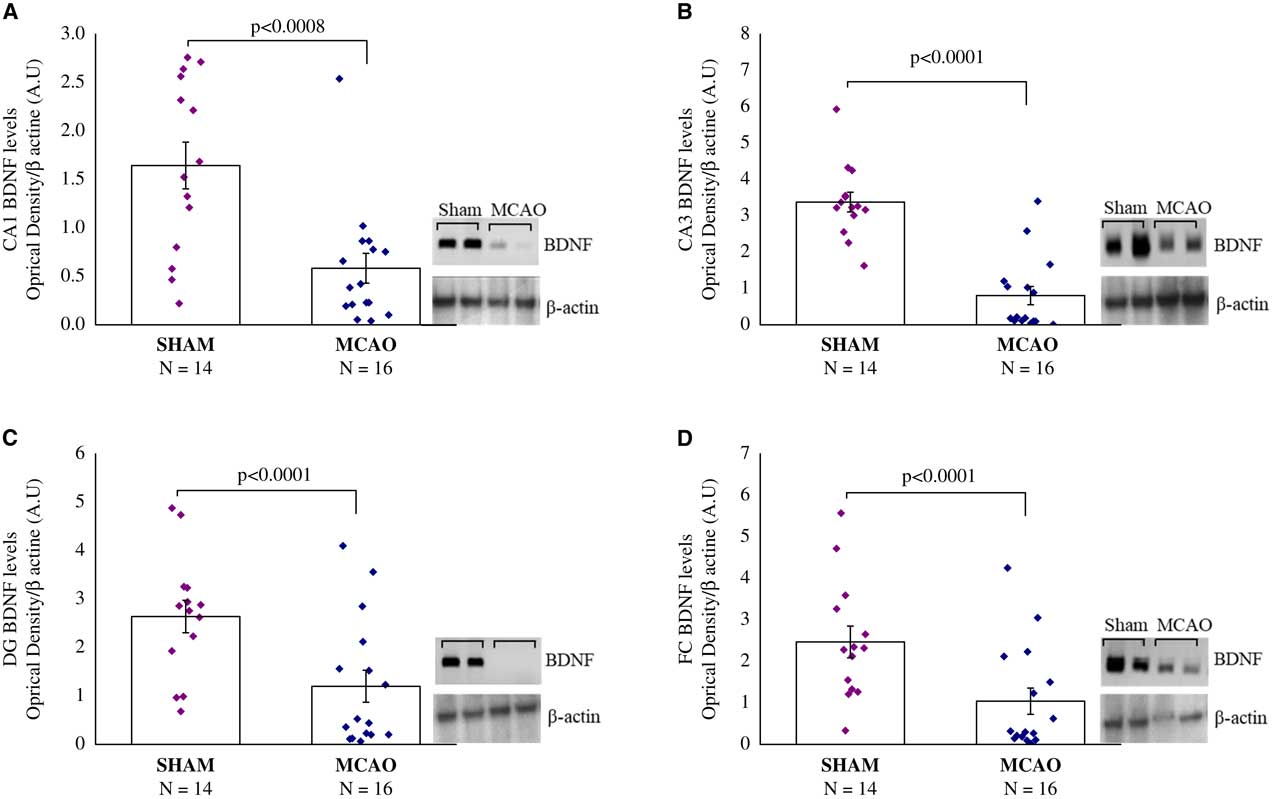
Figure 4 Brain-derived neurotrophic factor (BDNF) protein levels, 30 days after middle cerebral artery occlusion (MCAO) (n=16) or sham (n=14). Rats were sacrificed and their brains were removed for Western blotting analysis 24 hours following the last behavioral tests. Western blot analysis of BDNF protein levels and β-actin levels in representative gels and densitometry values of BDNF protein levels in the hippocampus sub-regions of Cornu Ammonis (CA1) (A), CA3 (B), and dentate gyrus (DG) (C), and in the frontal cortex (FC) (D) in the MCAO and sham rats. MCAO animals displayed significantly lower BDNF protein levels compared with sham rats. Densitometry values are means of 4–6 gels. All data represent group mean±SEM.
Brain-derived neurotrophic factor protein levels, 30 days after MCAO: in the hippocampal CA1, CA3 and DG sub-region, and in the frontal cortex (FC), there were significant differences in BDNF protein levels among groups (F(1,28)=14.5, p<0.0008; F(1,28)=48.3, p<0.0001; F(1,28)=11.6, p<0.0001: F(1,28)=8.4, p<0.008), respectively) (Figures 4A-4D). In all areas, MCAO animals displayed significantly lower BDNF protein levels compared with sham-operated rats (p<0.001) (Figures 4A-4D).
Unsupervised Fuzzy Clustering
The UFC method was applied to ten sets of both behavioral and molecular parametric data collected from 30 rats (Figures 5 and 6). The full range of the data set was classified into three (C=3) clusters. As shown in Figure 5, about 56% (56.25%) of the MCAO population was associated to cluster C1 (MCAO with depression-like symptoms), whereas 43.5% from among the MCAO animals were associated with cluster C3 (MCAO without depression-like symptoms). In the sham-control group, no rat was allocated to cluster C1 or cluster 3, and all the animals were associated with cluster C2 (control). Cluster C1 corresponds to the MCAO operated animals and is characterized by higher levels of depression, namely the PSD animals. The middle cluster (cluster C2) describes the sham-operated animals associated with un-stressed and un-depressed animals and is characterized by very low levels of depression. Cluster C3 corresponds to the MCAO operated animals and is characterized by some degree of depression levels (Figure 6).
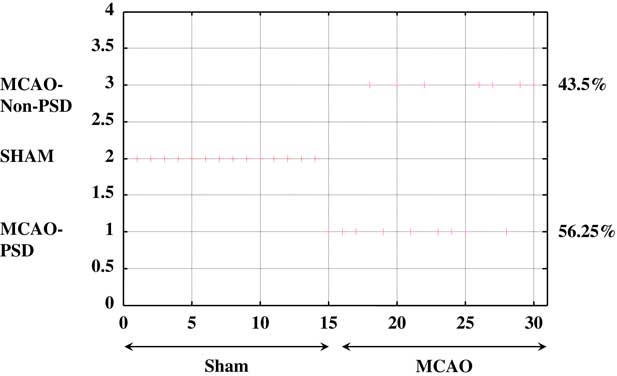
Figure 5 Unsupervised fuzzy clustering: hard classification of all rats to the partitioning cluster number. When “+” is attached to a specific number, it means that the maximum membership of this rat belongs to the corresponding cluster number. MCAO=middle cerebral artery occlusion; PSD=post-stroke depression.
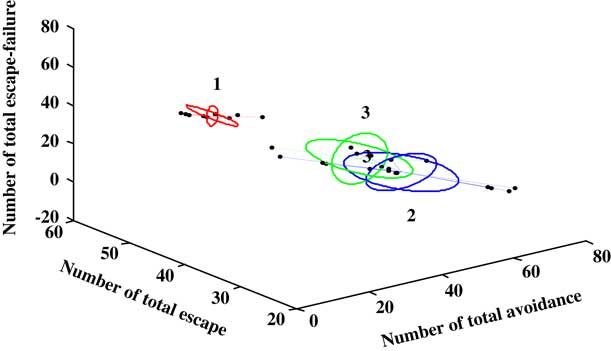
Figure 6 Only a three-feature space (number of total avoidance, number of total escape, and number of total escape-failure) of the ten behavioral and molecular parameters is presented. A line connects each point to its cluster centroid. The ellipses represent the standard error of each characteristic in each cluster. The unsupervised fuzzy clustering algorithm yielded clearly discrete clusters: the first cluster (1) is related to depressive behavior associated with middle cerebral artery occlusion (MCAO), and is characterized by very high levels of depression. Cluster (3) corresponds to the MCAO animals but is characterized by lower levels of depression, namely the MCAO-none-post-stroke depression (PSD) animals. The middle cluster (Cluster 2) represent the sham-operated animals.
Two-Way Shuttle Avoidance Learning
The centroid of cluster I (Table 2) represents a significantly low mean avoidance shuttles, as compared with cluster II and cluster III (one-way ANOVA: F(2,27)=116.6, p<0.00001). The centroid of cluster I also represents higher rates of escape failures as compared with the centroid of cluster II that represents significantly decreased rates of escape-failure (p<0.00001) and as compared with the centroid of cluster III (p<0.00001) (one-way ANOVA: F(2,27)=85.2, p<0.0001). The centroid of cluster III represents the mean between the two other clusters. The centroid of clusters I and III represents a significantly low number of exploratory shuttles as compared to cluster II (p<0.0001 for both groups) (F(2,27)=45.8, p<0.00001).
Table 2 The mean of the various centroid parameters in the ten behavioral and molecular parameters divided into three clusters
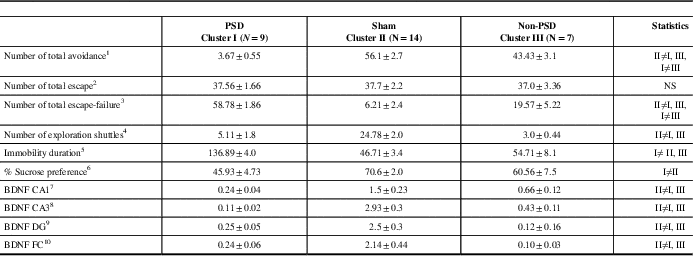
All data represent group mean±SEM.
PSD=post-stroke depression; BDNF=brain-derived neurotrophic factor; DG=dentate gyrus; CA=Cornu Ammonis; FC=frontal cortex.
1 F(2,27)=116.6, p<0.0001.
2 NS.
3 F(2,27)=85.2, p<0.0001.
4 F(2,27)=45.78, p<0.0001.
5 F(2,27)=109.45, p<0.0001.
6 F(2,27)=9.5, p<0.0008.
7 F(2,27)=11.8, p<0.00025.
8 F(2,27)=44.45, p<0.0001.
9 F(2,27)=32.3, p<0.0001.
10 F(2,27)=10.9, p<0.00035.
Sucrose Preference Test
The centroid of cluster I represents a significantly lower sucrose preference as compared with cluster II and cluster III (F(2,27)=9.5, p<0.0008).
Porsolt Forced-Swim Test
The centroid of cluster I represents a significantly higher immobility duration as compared with cluster II and cluster III (F(2,27)=109.4, p<0.00001).
Brain-Derived Neurotrophic Factor Protein Levels
The centroid of cluster I represents a significantly lower BDNF protein level in either of the CA1, CA3, and DG sub-regions as compared with clusters II and III (p<0.01 for both) (CA1: F(2,27)=11.5, p<0.00003; CA3: F(2,27)=42.1, p<0.0001; DG: F(2,27)=14.9, p<0.0005). The centroid of cluster I represents a significantly lower BDNF protein level in the FC area as compared with clusters II and III (p<0.001 for both) (F(2,27)=10.2, p<0.0005).
Discussion
Although the cerebral vascular accident is a frequent clinical problem, the etiology of the common behavioral consequences such as depression remains controversial and appears to be biological and psychosocial in origin, alone or in combination. In an attempt to study experimental PSD, we have surgically occluded the MCA in rats and found, during the 30-day postoperative period, behavioral and molecular changes. The presented study demonstrates, that more than 50% of animals that survived an ischemic stroke developed a complex of depressive-like behavior and reduced BDNF protein levels in the frontal cortex and hippocampal areas contralateral to the ischemic injury.
Several tests were used, to evaluate depressive-like behavior, assessing different aspects of depression—immobility, anhedonia, and learning and memory impairment: the Porsolt forced-swim test, sucrose preference test, and the two-way shuttle avoidance task were all employed 30 days post-surgery. These tests are commonly used in animal models of depression.Reference Cryan, Markou and Lucki 45 , Reference Nestler, Gould and Manji 46 It is known that behavioral tests, such as Porsolt swim test or the two-way shuttle avoidance task, can be affected by abnormal motor abilities following MCAO surgery. Although we did not find the MCAO group exhibiting less total escape behavior when compared with the sham-operated group, we confirmed our results using a behavioral test not affected by motor deficiencies, the sucrose consumption test. The integration of the three behavioral tests, together with the consistent results obtained, reinforces the predictive validity of our findings.
The animals that had undergone MCAO surgery were shown to have significantly more escape failures, reduced number of total avoidance shuttles, a significant elevation in immobility duration, and reduced sucrose preference compared with sham-operated animals. Moreover, a significant downregulation of BDNF protein levels in hippocampal sub-regions and frontal cortex were observed in all affected MCAO animals.
In the clinical arena, retrospective and prospective epidemiological studies of stroke patients indicate, that only a proportion of the population after stroke will develop a depressive disorder. Animal behavioral studies, however, have generally tended to overlook this aspect and have commonly regarded the entire group of animals subjected to certain study conditions as homogeneous. Thus, in an attempt to develop animal models of long-term chronic behavioral changes in a comparable manner to human diagnosis, the mathematical model dynamic, unsupervised, hierarchical fuzzy clustering was used to further assess the individuals that developed depressive symptoms following MCAO operation, that is, PSD. Unsupervised fuzzy clustering analysis is a mathematical technique that groups together objects in the multidimensional feature space according to a specified similarity measurement, thereby yielding clusters of similar data points, which can be represented by a set of prototypes or centroids.Reference Gath and Geva 42 , Reference Cohen, Zohar, Matar, Kaplan and Geva 44 , Reference Geva 47 - Reference Geva and Pratt 49 In fuzzy clustering, each data point is attached to each cluster with different degrees of membership. After the clustering procedure, each cluster is labeled by using its centroid features. This can be a useful means for identifying meaningful structures among multidirectional data, by which it is possible to unravel relationships among different parameters and expose new information and models in the global data.Reference Gath and Geva 42 , Reference Cohen, Zohar, Matar, Kaplan and Geva 44 , Reference Geva 48 , Reference Geva and Pratt 49 The UFC analysis focused on the heterogeneous nature of the behavioral changes from MCAO surgery.
When behavioral and molecular data for the entire MCAO sample were examined by this means, it was demonstrated that about 56% develop significant behavioral disruptions associated with depressive symptoms. Epidemiological studies found that 18%-60% of human stroke survivors developed depression.Reference Astrom, Adolfsson and Asplund 20 , Reference Berg, Palomäki, Lehtihalmes, Lönnqvist and Kaste 39 , Reference Kotila, Numminen, Waltimo and Kaste 50 , Reference Ramasubbu, Robinson, Flint, Kosier and Price 51 The considerable variability between studies can be attributed to numerous factors among those methodological differences regarding the patient selection, setup, and timing of the clinical assessment and the lack of standardized diagnostic criteria. Thus, the prevalence of PSD in our study model seems to fit into the known human prevalence pattern.Reference Astrom, Adolfsson and Asplund 20 , Reference Berg, Palomäki, Lehtihalmes, Lönnqvist and Kaste 39 , Reference Kotila, Numminen, Waltimo and Kaste 50 , Reference Ramasubbu, Robinson, Flint, Kosier and Price 51
Converging lines of evidence implicate the neurotrophic BDNF in the pathophysiology of depression. Decreased BDNF has been demonstrated in animal models and patients with depression.Reference Duman 24 , Reference Nibuya, Morinobu and Duman 52 , Reference Kronenberg, Gertz, Heinz and Endres 53 In contrast, antidepressant treatments almost universally promote neurogenesis and neurotrophic factor gene expression.Reference Kronenberg, Gertz, Heinz and Endres 53 , Reference Malberg, Eisch, Nestler and Duman 54 Therefore, our findings suggest that as far as BDNF is concerned, PSD is very similar to other mood disorder, and is associated with reduced BDNF brain levels.
Clinically, PSD is associated with an increased disability,Reference Schwartz, Speed, Brunberg, Brewer, Brown and Greden 55 increased cognitive impairment,Reference Kauhanen, Korpelainen and Hiltunen 56 increased mortality, both on short- and long-term,Reference Morris, Robinson, Andrzejewski, Samuels and Price 57 , Reference Williams, Ghose and Swindle 58 increase risk of falls,Reference Jorgensen, Engstad and Jacobsen 59 and finally, PSD impedes the rehabilitation and recovery process, and compromises quality of life.Reference Paolucci, Antonucci and Grasso 60 This association of PSD and poor clinical outcome can be explained in several ways. The poor clinical condition can cause PSD, PSD can lead to a poor outcome, or a third parameter may lead to both. It is possible that reduced activation of the BDNF neuroprotective pathway may lead to a poor clinical outcome and to PSD. If that is the case, PSD may reflect a failure of activation of neuroprotective mechanisms. This hypothesis was partially supported by the observation that serum concentrations of BDNF increase following an acute ischemic stroke but they decrease in stroke survivors who subsequently develop PSD.Reference Zhou, Lu and Xu 61
Kronenberg et al.Reference Kronenberg, Gertz, Heinz and Endres 53 reported earlier investigations in the relationship between the left-sided MCAO and the right-sided MCAO, the influence of age on the development of post-stroke depressive behavior, and the gender derivative and the effect of a combination of stress and stroke on the mode of disorders and social behaviors. Our animal model of PSD, in which PSD was induced without further stress besides the stroke itself, can serve in the future as an assessment tool for future therapies for PSD. It is well known that selective serotonin reuptake inhibitors, and tricyclic antidepressants, stimulants, and even electroconvulsive therapy have shown benefit in the treatment of PSD.Reference Andersen, Vestergaard and Lauritzen 62 - Reference Wiart, Petit, Joseph, Mazaux and Barat 65 The role of prophylactic antidepressant treatment is unclear, and so is the role of anticonvulsants and SNRI’s in the treatment of PSD. Our model could potentially contribute important preclinical data on the efficacy of those therapeutic measures, as well as other acute BDNF levels modifying modalities on stroke clinical outcome and on PSD. Animal behavioral studies, however, have generally tended to overlook this aspect and have commonly regarded the entire group of animals subjected to certain study conditions as homogeneous.
Acknowledgments
The authors gratefully acknowledge Dr. M. Dubilet, Resident, Division of Anesthesiology, Soroka Medical Center, for his help in the laboratory, as well as in the histology analysis. The support of Shira Ovadia, Director of Animal Resources Unit, is also gratefully acknowledged. Many thanks to the staff at the Critical Care Unit, Soroka Medical Center, for their support and helpful discussions. The data obtained are part of Ruslan Kuts’s PhD thesis.
Statement of Authorship
GI came up with the general idea for the experiment, provided financial support (academic sources), and partook in the article. MB was responsible for logistics and surgical procedures. DF was responsible for anesthetic procedures and took part in conducting behavioral tests (sucrose and forced-swim tests). HNS planned the experiment, processed results, partook in manuscript writing, prepared the manuscript for publication, and coordinated between coauthors. JG prepared rat brain for experimental procedures, evaluated the NSS (blinded investigator), stained the rat brain, quantified the brain edema, and calculated the infarct zone. RK carried out the behavioral tests (sucrose and forced-swim tests). VZ processed behavioral test results, assisted in conducting operations on rats, evaluated the NNS (blinded investigator), and partook in behavioral testing (sucrose and forced-swim tests). ABG statistically processed experimental data, participated in evaluating the obtained results, and took part in writing the article. ZK was part of Ms. Cohen’s research group and participated in conducting tests (BDNF, Western blot analysis, and two-way shuttle avoidance learning). HC supervised the histological and behavioral experiments (BDNF, Western blot analysis, and two-way shuttle avoidance learning) carried out in her laboratory.
Disclosures
GI, MB, DF, HNS, JG, RK, AG, ZK,VZ, and HC have nothing to disclose.










