Introduction
Cerebral radionecrosis is a delayed complication of cranial irradiation. It tends to occur between 2 and 32 months after radiation Reference Shah, Vattoth and Jacob1 . Tissue necrosis is more likely to occur with higher doses per fraction and with prior radiation to the region Reference Ruben, Dally, Bailey, Smith, McLean and Fedele2 . It can occur in the setting of brain tumors (such as glioma, meningioma, or brain metastases). It can also occur following the treatment of tumors such as nasopharyngeal carcinoma or clival chordoma that are adjacent to the brain. Depending on the location of the radionecrosis, different clinical symptoms and signs can occur, and these can be quite disabling. A definitive diagnosis of radionecrosis requires histological necrosis in the right clinical context. Presumptive diagnoses can be made based on history and advanced neuroimaging.
The main treatment for symptomatic cerebral radionecrosis is dexamethasone, and while often effective, it causes serious side effects when used long term. Surgical options include resection of the region of radionecrosis or laser interstitial therapy. Hyperbaric oxygen therapy has possible therapeutic value Reference Co, De Moraes and Katznelson3 . Finally, bevacizumab, an intravenous monoclonal antibody to vascular endothelial growth factor, is often prescribed off-label for cerebral radionecrosis.
The quality of evidence for bevacizumab for cerebral radionecrosis is limited. In 2009, Levin et al. Reference Levin, Bidaut and Hou4 conducted a randomized trial of 14 patients with cerebral radionecrosis. None of the patients had brain metastases. The patients were randomized to bevacizumab versus placebo. The MRI scans improved with a mean percentage reduction in T1 gadolinium-enhancing volume of 59% and 63% on T1 and fluid-attenuated inversion recovery (FLAIR) images, respectively. There was a reduction in steroid use, but no clear improvement in symptoms. There were two serious, related adverse events: pulmonary embolism and cerebral venous sinus thrombosis. In 2013, Boothe et al. Reference Boothe, Young, Yamada, Prager, Chan and Beal5 conducted a retrospective review of 11 patients with radionecrosis in the context of prior stereotactic radiosurgery for brain metastases. These patients were treated with bevacizumab, and they had improved imaging and reduced steroid requirements. All but one patient had improved symptoms. In 2018, Xu et al. Reference Xu, Rong and Hu6 conducted a randomized trial of 112 patients with bitemporal radionecrosis following treatment for nasopharyngeal carcinoma. They reported improvement with bevacizumab compared with steroids. Of the 38 patients in the bevacizumab group whose imaging improved, 31 (81.58%) experienced improved symptoms. Among 17 patients in the corticosteroid group whose imaging improved, 15 showed improved symptoms. A recent systematic review found 21 studies with a total of 115 patients where bevacizumab was used for cerebral radionecrosis Reference Delishaj, Ursino and Pasqualetti7 . Most of these were retrospective cohort studies. The most frequent bevacizumab dose was 7.5 mg/kg every 2 weeks for 4 cycles. There was consistent reduction in T1 and T2/FLAIR changes after bevacizumab therapy. Steroid doses often decreased after bevacizumab. Symptom improvements were not systematically documented.
Increasingly, bevacizumab is being employed to treat cerebral radionecrosis; however, the magnitude of clinical benefits and full range of toxicities remains unclear. The goal of our research study was to review our experience using bevacizumab for cerebral radionecrosis, with a focus on radiographic changes, symptoms, performance status, steroid use, and adverse events.
Methods
Following institutional research ethics board approval, the charts of neuro-oncology patients treated with bevacizumab between January 2017 and March 2021 were reviewed. All patients were treated at Princess Margaret Cancer Centre in Toronto, Canada. Two authors (SAC, RCR) extracted the clinical information from a database. Diagnosis of radionecrosis was based on clinical features, imaging, and/or pathology. A diagnosis of radionecrosis was only considered in patients who had received high-dose cerebral radiotherapy within 3 months, up to a few years. In most patients, advanced imaging techniques like perfusion or spectroscopy were used to support a diagnosis of radionecrosis over tumor progression. Patients with glioma were excluded because bevacizumab can be used to treat both tumor progression and radionecrosis in these patients. We recorded change in symptoms, change in steroids, change in performance status, time to tumor progression, and time to death. Performance status was estimated just before bevacizumab therapy, and the best performance status at subsequent follow-up visits was that patient’s post-bevacizumab performance status. Patient-reported performance status was taken, where available, from Edmonton Symptom Assessment System (ESAS) self-reports. We delineated the volume of necrosis pre- and post-bevacizumab on T1-post-gadolinium and FLAIR MRI scans. It should be noted that the T1-weighted sequences estimate the effects of radiation necrosis, recognizing that it is a surrogate of blood–brain barrier and blood–tumor barrier integrity. The last MRI before bevacizumab was compared to the best MRI after bevacizumab. T1-post-gadolinium and FLAIR MRI images were imported to RayStation treatment planning system (v8, RaySearch Laboratories AB, Stockholm, Sweden) and contoured and reviewed by two authors in order to estimate the volume of cerebral necrosis before and after the administration of bevacizumab (PAJ, DBS).
Statistical analysis included paired two-sided t-tests for change in T1 gadolinium-enhancing volume and for change in Eastern Cooperative Oncology Group performance status. Kaplan–Meier estimators were used to plot overall survival (OS) and progression-free survival (PFS). PFS was defined as time from first bevacizumab treatment to cancer progression (by imaging or pathology) or death.
Results
Fifteen patients were treated with bevacizumab for non-glioma cerebral radionecrosis during the study period. Of these, eight had brain metastases, six had meningiomas (grades ranging from 1 to 3), and one had nasopharyngeal carcinoma. The median age was 55 years. The median Eastern Cooperative Oncology Group (ECOG) performance status was 2. There were ESAS data available for 10 of the 15 patients, but pre- and post-bevacizumab ESAS data were only available for three patients. One of those three patients had a pre-bevacizumab self-reported ECOG score of 1 ± 0 (mean ±, standard deviation), and post-bevacizumab, it was 3 ± 0; the second patient’s scores were 1.3 ± 1.1, then 2.5 ± 0.7; the third patient’s scores were 0.7 ± 0.6, then 1.3 ± 0.5. The median central nervous system necrosis grade (Common Terminology Criteria for Adverse Events version 5.0) was 2. Two-thirds of patients had radionecrosis in an eloquent portion of the brain. Two patients had radiation-induced meningiomas in the context of prior childhood radiotherapy for hematological malignancy. The median cumulative brain parenchyma biological effective dose (BED) with α/β = 2 was 86.40 Gy, whereas the median tumor BED was 127.50 Gy, using α/β ratio of 3 for meningioma and 10 for brain metastases and nasopharyngeal carcinoma (Table 1).
Table 1: Patient baseline characteristics (N = 15)

Acronyms: Eastern Cooperative Oncology Group (ECOG), Common Terminology Criteria for Adverse Events (CTCAE), stereotactic radiosurgery (SRS), biologically equivalent doses (BED).
* Tumor BED α/β ratio of 3 for meningioma, 10 for the other tumors.
** Brain BED α/β ratio of 2.
All were treated with bevacizumab 7.5 mg/kg, with planned doses every 3 weeks. Most patients received four total doses of bevacizumab. Nine patients were undergoing treatment with dexamethasone when bevacizumab was started, and five of these patients reduced their dexamethasone dose during bevacizumab therapy. Patients who were on high-dose dexamethasone prior to bevacizumab had steroid side effects like weight gain, insomnia, and hyperglycemia. Most patients had no change to their ECOG performance status (paired t-test p-value = 0.7, Figure 1); median ECOG performance status was 2 both before and after bevacizumab. Post-bevacizumab performance status was typically measured at the 3-month follow-up visit. One patient’s hemiparesis improved to the point that they were able to walk again. There were subtle clinical responses in six patients. Two patients’ hemiparesis improved slightly: (1) before bevacizumab: left pronator drift and spasticity; after bevacizumab: no pronator drift, but ongoing spasticity; (2) before bevacizumab: right distal 4/5 strength; after bevacizumab: right pronator drift. One patient’s numbness improved. Adverse events potentially ascribable to bevacizumab occurred in four patients. One patient within meningioma had scalp wound dehiscence, but there were signs of infected, growing tumor even before the bevacizumab. One patient had a sudden, unexpected death at home, but no subsequent autopsy. One patient had a leg deep vein thrombosis (DVT). Another patient had a DVT in addition to a pulmonary embolism and ischemic stroke (Table 2).
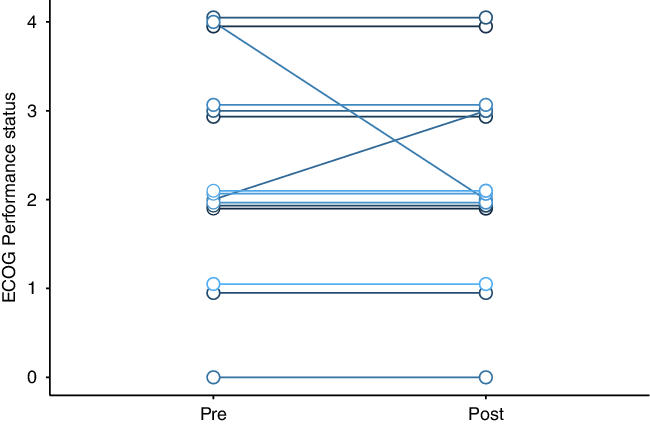
Figure 1: Change in cerebral radionecrosis patients’ Eastern Cooperative Oncology Group (ECOG) performance status after bevacizumab therapy.
Table 2: Treatment and clinical response (N = 15)
Patients treated with bevacizumab had reductions in necrosis and edema volumes, assessed by T1-post-gadolinium and FLAIR volumes, compared to their pre-bevacizumab baseline (Figure 2). For T1, the average reduction in volume was 3.0 cm3 (95% CI −4.9 to 11.0 cm3), and for FLAIR, the average reduction in volume was 27.9 cm3 (95% CI −12.0 to 67.7 cm3). The median T1 volume went from 16.9 cm3 to 12.5 cm3 after bevacizumab, and the median FLAIR volume went from 55.0 cm3 to 24.2 cm3. One brain metastasis in a patient with metastatic melanoma who was initially thought to have cerebral radionecrosis grew during treatment with bevacizumab and repeat surgery confirmed viable tumor. When omitted from the statistical analysis, for T1, the average reduction in volume was 5.7 cm3 (95% CI −0.3 to 11.7 cm3, p = 0.06), and for FLAIR, the average reduction in volume was 43.5 cm3 (95% CI 20.3 to 66.8 cm3, p = 0.001). An analysis of the correlation between time from radionecrosis detection to bevacizumab and percent reduction in T2 volume in this subset revealed a correlation coefficient of 0.45, indicating more imaging response in patients treated sooner.
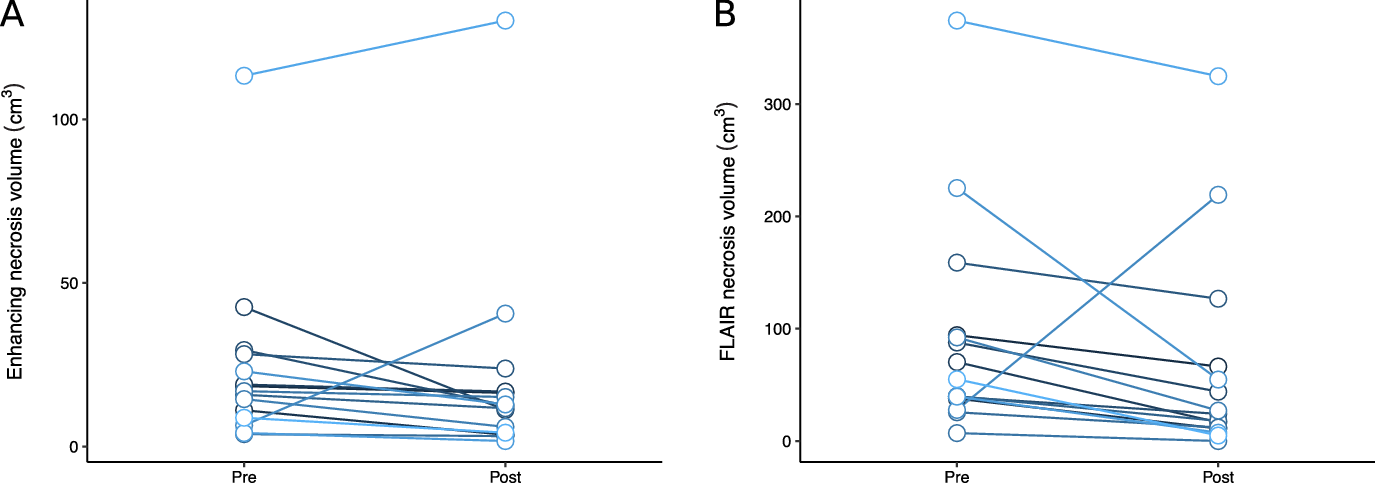
Figure 2: Change in patients’ radionecrosis volumes on T1-post-gadolinium (A) and FLAIR (B) images after bevacizumab therapy.
The median OS for these bevacizumab-treated patients was 21.7 months (Figure 3, Panel A) from the time of first bevacizumab administration. The median OS was 28.5 months from the time of first MRI showing radionecrosis. Nine patients had tumor progression during the follow-up period. The median PFS was 6.5 months (Figure 3, Panel B) where PFS is defined as freedom from tumor progression, not necrosis progression. The median OS for meningioma patients was not reached but their median PFS was 6.6 months. The median OS and PFS for metastasis patients were 21.7 and 6.4 months, respectively. One patient later underwent a second course of bevacizumab (4 more treatments) for radionecrosis, but did not respond the second time around, and repeat surgery confirmed tumor progression. Five patients (three with metastases and two with meningioma) are alive without tumor progression. The median follow-up time is 14.2 months.

Figure 3: Overall survival (A) and progression-free survival (B) of radionecrosis patients from first bevacizumab therapy. Progression-free survival was defined as time to death or tumor progression.
Discussion
In this single-center retrospective review of bevacizumab-treated non-glioma cerebral radionecrosis patients, bevacizumab improved radiographic findings but only minimally improved clinical symptoms. Treatment was not associated with an improvement in performance status and was likely associated with several significant adverse events and only some steroid dose reductions. Most patients had progression of their underlying malignancy within 6.5 months of receiving bevacizumab, further reducing the benefit of this intervention on patient outcomes. Our cohort includes a heterogeneous group of patients, so the OS and PFS numbers should be interpreted only insofar as they limit the duration of potential quality of life benefit from bevacizumab.
Our data accord with previously published results showing that bevacizumab can reduce cerebral edema and improve the appearances of lesions on T2- and T1-based post-gadolinium scans Reference Delishaj, Ursino and Pasqualetti7,Reference Khan, Zhao, Arooj and Liao8 . Our cohort is primarily made up of patients with meningioma and brain metastases. Brain metastases patients were not represented in the Levin et al. randomized trial of bevacizumab for radionecrosis Reference Levin, Bidaut and Hou4 . There have been at least two systematic reviews of bevacizumab for radionecrosis. In one, only brain metastasis patients were included and imaging responses were seen in 93% of patients, 47% T1-post-gadolinium volume reduction, and 62% FLAIR volume reduction on average Reference Khan, Zhao, Arooj and Liao8 . In another systematic review, patients with brain metastasis, meningioma, and nasopharyngeal carcinoma were included Reference Delishaj, Ursino and Pasqualetti7 . They found T1-post-gadolinium reductions of 60% and T2/FLAIR reductions of 64%.
The rate of potential bevacizumab side effects was higher in our patients than in another similar cohort Reference Sadraei, Dahiya and Chao9 . In particular, we found that 2 of 15 patients (14%) developed a DVT. Among 3763 patients with metastatic colon cancer treated with chemotherapy ± bevacizumab, the rates of wound healing issues were 0.4% without bevacizumab versus 0.9% with bevacizumab. Venous thromboembolism occurred in 6.5% versus 8.2% with bevacizumab Reference Hurwitz, Tebbutt and Kabbinavar10 . These trial data are similar to real-world data showing the risk of venous thromboembolism with bevacizumab was 5% in Spain Reference Marín-Pozo, Duarte-Pérez and Sánchez-Rovira11 . The slightly higher rates of adverse events in our cohort can easily be explained by random effects, given the small sample size.
Several studies have reported on the clinical benefits of bevacizumab for radionecrosis. Of the seven melanoma patients who received bevacizumab for cerebral radionecrosis, all had “clinical improvement” Reference Glitza, Guha-Thakurta and D’Souza12 . Of 11 brain metastasis patients who received bevacizumab, there was symptomatic improvement in 7 of 11, no change in symptoms in 3 of 11, and worsening in 1 of 11 Reference Boothe, Young, Yamada, Prager, Chan and Beal5 . In another case series of brain metastasis patients, there was clinical improvement in 11 of 14 patients Reference Zhuang, Yuan and Zheng13 . In a phase 2 trial of low-dose bevacizumab for radionecrosis, 17 of 21 patients had some clinical improvement Reference Zhuang, Yuan and Zheng13 . Our study found only 6 of 15 patients had clinical improvement, and for most of these patients, the improvement was modest.
Performance status has been used before to quantify clinical improvement with bevacizumab. Of 13 brain metastasis patients who received bevacizumab, the median Karnofsky performance status (KPS) was 80 before treatment and remained 80 at subsequent follow-ups till it fell to 60 after 6 months Reference Sujijantarat, Hong and Owusu14 . In an individual patient data meta-analysis of 54 brain metastasis radionecrosis patients, only 10 had KPS reported Reference Khan, Zhao, Arooj and Liao8 . Of these 10 patients, 8 had an improvement in KPS after bevacizumab.
A main strength of our study is that we quantified performance status, albeit retrospectively, to better measure the potential clinical benefit from bevacizumab. We measured radionecrosis volumetrically to better quantify the degree of radiological improvement. However, our study has significant limitations. Data are from one institution, and the sample size is small. Patient performance status was not consistently reported prospectively, and most scores were imputed from the clinical notes. Further, ECOG performance status is graded coarsely so clinically meaningful improvement might not be captured on this scale. Finally, we selected patients with significant radionecrosis for bevacizumab therapy from a larger cohort of patients with presumed cerebral radionecrosis, many of whom had minimal symptoms and lesser degrees of radionecrosis. It is conceivable that better outcomes might have been observed in these patients, had they received bevacizumab.
The results of our study are quite different from earlier published reports that suggest that bevacizumab is an effective drug for the management of cerebral radionecrosis. In contrast to prior studies, our study reports limited clinical benefit and probably toxicities of bevacizumab when used for cerebral radionecrosis. There are a few possible reasons for these discrepant results. Many previously published case series focused mostly on imaging rather than clinical outcomes Reference Delishaj, Ursino and Pasqualetti7,Reference Khan, Zhao, Arooj and Liao8 . Conceivably, our patients may have had radionecrosis that was too advanced to benefit from bevacizumab. Time from radiation to bevacizumab is unlikely to explain this effect, though; in the Levin et al. randomized trial, the average time from radiation to bevacizumab was 3 years, compared with 1.5 years in our series Reference Levin, Bidaut and Hou4 . The interpretation and reporting of minor clinical responses can also explain some discrepancies. There may be a publication bias at play: in an individual patient data meta-analysis of 54 brain metastasis radionecrosis patients, only 10 had KPS reported Reference Khan, Zhao, Arooj and Liao8 . Our cohort included many patients with necrosis in an eloquent portion of the brain, perhaps reducing the potential benefits of bevacizumab. Our cohort included many patients with necrosis in an eloquent portion of the brain, perhaps reducing the potential benefits of bevacizumab. Our cohort especially included patients with advanced metastatic disease who are at risk of the medical complications associated with bevacizumab use. Pre-radiation or intra-radiation bevacizumab dosing might be more effective at preventing radiation necrosis in the setting of high-risk radiation treatments. Our study looked at potential bevacizumab adverse events, but did not systematically look at the adverse effects caused by dexamethasone nor did it compare the rates of bevacizumab side effects to other cohorts of cancer patients receiving bevacizumab. This limits the interpretation of adverse event rates. Lastly, random effects can be important with small sample sizes.
In conclusion, in this retrospective cohort of bevacizumab-treated cerebral radionecrosis patients, bevacizumab was associated with improvement in MRI measures, but no commensurate improvement in clinical measures and some concerning toxicities. Our results should be of use to clinicians who are considering bevacizumab for patients with cerebral radionecrosis. Although not addressed in this study, bevacizumab is an expensive drug; a pharmacoeconomic analysis of the benefits of this agent for cerebral radionecrosis may further dampen enthusiasm for use in this setting. A large phase II study randomizing patients with cerebral radionecrosis following radiosurgery for cerebral metastases to corticosteroids versus bevacizumab recently closed due to poor accrual (clinicaltrials.gov NCT02490878). If completed, this trial would likely have provided definitive evidence for or against the use of bevacizumab for cerebral radionecrosis. Nonetheless, limited data from this trial are anticipated and will further illuminate the role of bevacizumab for this complication of radiotherapy.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/cjn.2022.64.
Conflicts of Interest
None.
Statement of Authorship
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by SAC, RCR, PAJ, and DBS. The first draft of the manuscript was written by SAC and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.







