Introduction
Multiple sclerosis (MS) is a chronic autoimmune demyelinating inflammatory disease of the central nervous system (CNS), in the majority of cases gradually leading to progressive, severe disability if left untreated. MS is the leading cause of non-traumatic disability among young adults in the developed world. It is most often diagnosed between 20 and 40 years of age and affects women and men at a ratio of approximately 2:1. Reference Yeshokumar, Narula and Banwell1–Reference Filippi, Bar-Or and Piehl3 The clinical course of MS can be characterized as (i) clinically isolated syndrome (CIS), (ii) relapsing-remitting (RRMS), (iii) primary progressive (PPMS), or (iv) secondary progressive (SPMS). Each of the above MS categories can be further subcategorized as either active or inactive, based on both the clinical relapse rate and MRI findings (new T2 lesions and/or active, gadolinium-enhancing lesions-GdELs). Further, progressive forms can be subcategorized as actively progressive or stable. Reference Lublin4
Significant progress in understanding MS pathophysiology has been accomplished in the past decades. Two hundred and thirty-three genetic variants have been identified as risk factors for MS, 32 of which refer to the major histocompatibility complex family (MHC). 5 Prominent among the many risk variants, MHC Class II DR15 molecule entails mechanistically relevant susceptibility to the disease rather than just being a genetic marker. Reference O’Connor, Bar-Or and Hafler6 Additional genetic variants associated with the disease refer to other genes of the immune system, such as genes involved in T-cell activation and proliferation (IL-2, IL-7R), tumor necrosis factor-alpha (TNF-α)-related pathways, and vitamin D metabolic pathways (GC, CYP24A1). Reference Filippi, Bar-Or and Piehl3,Reference De Jager, Jia and Wang7–Reference Mokry, Ross and Ahmad11
In MS, myelin is phagocytosed by CD68-positive macrophages, while immune cells including B and T cells seem to be activated in the periphery and to express adherence molecules that enable them to cross blood-brain barrier (BBB) in order to participate in the formation of MS lesions. Accumulating evidence suggests an important contribution of CD4+ T cells to disease pathophysiology. Reference Ota, Matsui, Milford, Mackin, Weiner and Hafler12 Often being present from the beginning and increasing in quantity as disease progresses, axonal degeneration is regarded as a correlate of disability progression. Reference Bjartmar, Yin and Trapp13
MS was long considered mainly T-cell-mediated; however, intrathecal IgG synthesis, Reference Bonnan14 a hallmark of MS, supports B-cell involvement. Reference Cepok, Rosche and Grummel15,Reference Kowarik, Cepok and Sellner16 The T-cell-dominated view of MS pathogenesis was further challenged by the remarkable efficiency of CD20+ B-cell depletion in eliminating inflammatory activity in patients with MS. In this review, we aim to shed light on the key role B cells play in the pathogenesis of MS and present current advances in MS treatment strategies based on promising and effective B-cell-targeted therapeutic regimens.
B Cells in MS Pathology
Histological studies of active MS lesions have demonstrated that B cells can reside in the perivascular space and the CSF but also within the parenchyma. Reference Krumbholz, Derfuss, Hohlfeld and Meinl17 Moreover, ectopic lymphoid follicles are found primarily in the intrameningeal spaces. Reference Kivisäkk, Imitola and Rasmussen18 and are associated with subpial cortical demyelination in patients with SPMS. Reference Moreno Torres and García-Merino19,Reference Lassmann20 In addition, four histopathological patterns have been proposed for the classification of acute MS plaques. Type I lesions (15% of MS patients) are characterized by a T-cell and activated microglia inflammatory environment without immunoglobulin deposition and complement activation. On the contrary, type II lesions (58% of MS patients) develop in an inflammatory milieu with immunoglobulin production and complement activation. Demyelination in type III lesions (26% of MS patients) is accompanied by oligodendrocyte apoptosis without immunoglobulin deposition or complement activation. Finally, in type IV lesions (rare; 1% of MS patients) inflammatory modulators result in nonapoptotic death of oligodendrocytes in the white matter surrounding the plaque due to metabolic disorganization processes. Reference Gh Popescu, Pirko and Lucchinetti21 However, it is important to note that IgG deposits in MS histopathological specimens are not specific for MS Reference Barnett, Parratt, Cho and Prineas22 and that no disease-characterizing autoantibodies have been defined to date. Nevertheless, pattern II has been linked to better response to plasma exchange. Reference Keegan, König and McClelland23
Potential Roles of B Cells in MS Pathophysiology
Several studies have explored potential roles of B cells in the development of MS: antibody production, antigen presentation, and secretion of pro- and anti-inflammatory mediators are three prominent research directions that have been explored. Reference Gasperi, Stüve and Hemmer24,Reference Häusser-Kinzel and Weber25 In addition, clear epidemiological associations of B-lymphotropic Epstein-Barr virus (EBV) infection to MS have led to the explorations of its pathophysiological relevance. Reference Guan, Jakimovski, Ramanathan, Weinstock-Guttman and Zivadinov26
Autoantibodies
Autoreactive B cells that escape peripheral tolerance checkpoint selection could target antigens of the CNS and cause autoimmune inflammation; however, no consistent B-cell antigen that is specific for MS and that causes demyelination has been identified to date despite numerous attempts. Intrathecal oligoclonal bands, a hallmark of MS diagnosis found in up to 95% of patients, Reference Abdelhak, Hottenrott and Mayer27,Reference Thouvenot28 are not specific for MS (found also in e.g. meningitis and subacute sclerosing panencephalitis) and have been found to target intracellular antigens in patients with MS. Reference Brändle, Obermeier and Senel29,Reference Willis, Stathopoulos, Chastre, Compton, Hafler and O’Connor30 In addition, detection of intrathecal IgM synthesis has been associated with onset of relapses and a more aggressive disease course. Reference Villar, Masjuan and González-Porqué31 Similarly to antibodies of oligoclonal bands, B cells of MS lesions have been found to target intracellular antigens. Reference Willis, Stathopoulos, Chastre, Compton, Hafler and O’Connor30 Antibodies previously thought to be present in MS such as antibodies against myelin oligodendrocyte glycoprotein (MOG) rather characterize a distinct disease entity (MOG-antibody disease) that encompasses pediatric acquired demyelinating syndrome, recurrent optic neuritis, acute disseminated encephalomyelitis, and neuromyelitis optica without anti-aquaporin four autoantibodies. A minority of MS patients harbor antibodies against a variety of antigens (some of them cell surface proteins) such as contactin-2, Reference Boronat, Sepúlveda and Llufriu32 OMGP, Reference Gerhards, Pfeffer and Lorenz33 and other peptide and lipid antigens. Reference Yeste and Quintana34,Reference Kanter, Narayana and Ho35
It must be noted that autoantibodies can also be responsible for the activation and chemotaxis of CD4+ T cells. The opsonization of myelin antigens, even at low concentrations, enhances the presentative competence of resident antigen-presenting cells (APCs), such as macrophages and dendritic cells, leading to increased recruitment of effector T cells and, consequently, aggravation of the disease severity, as explained in more detail below. Reference Trotter, DeJong and Smith36–Reference Getahun, Dahlström, Wernersson and Heyman38
B Cells as Antigen-Presenting Cells
B cells are efficient APCs and express MHC class II and costimulatory molecules, such as CD40, CD80, and CD86. Reference Häusser-Kinzel and Weber25,Reference Kinzel and Weber39 They can capture soluble and membrane-tethered antigens via their B-cell receptor (BCR) and present them to T cells in an up-to-a 10.000 times more efficient way compared to myeloid APCs. Reference Ancau, Berthele and Hemmer40 Evidence from experimental autoimmune encephalitis, a rodent model simulating "efferent" MS pathophysiology, opposes the hypothesis that the antigen-presenting function of B cells is central to the pathophysiology of MS. Specifically, MOG-specific B cells may initiate CNS inflammation and, consequently, the symptomatic onset of the disease, but do not affect either the proliferation or the molecular profile (i.e. secreted cytokines, activation markers) of MOG-specific T cells in the spleen and the draining lymph nodes. Reference Flach, Litke and Strauss37
On the other hand, a recently published report focusing on human leukocyte antigen (HLA)-DR15, which is the major genetic risk factor for MS, addresses how the immunopeptidomes presented by both DR15 allomorphs, DR2a and DR2b, on different APCs in the thymus, peripheral blood, and brain – including B cells – could affect autoimmune T cells. The results showed that DR2a and DR2b immunopeptidomes on B cells are significantly skewed toward HLA-DR-self peptides (HLA-DR-SPs) – compared to monocytes – which are consequently presented to autoreactive CD4+ T cells. These T cells responded robustly to individual and pooled HLA-DR-SPs in MS patients, compared to healthy donors, suggesting that DR2a and DR2b could jointly shape an autoreactive T-cell repertoire in MS. Reference Wang, Jelcic and Mühlenbruch41
B Cells as a Source of Cytokines
Physiologically, B cells can be a source of both proinflammatory and anti-inflammatory (regulatory) cytokines. B cells of RRMS patients however feature a profile that is skewed towards an abnormally hyperactive proinflammatory response. In mice, high levels of B-cell-secreted IL-6 can foster the differentiation of Th17 cells, while preventing the generation of T regulatory cells. Reference Bettelli, Carrier and Gao42–Reference Korn, Mitsdoerffer and Croxford44 In MS patients, B-cell production of lymphotoxin alpha (LT-α), TNF-α, and granulocyte macrophage-colony stimulating factor (GM-CSF) appears elevated, forging a chronically inflammatory milieu within the CNS. Reference Moreno Torres and García-Merino19,Reference Häusser-Kinzel and Weber25,Reference Li, Rezk and Miyazaki45 At the same time, anti-inflammatory, regulatory cytokines that are produced by B cells, such as IL-35, Reference Shen, Roch and Lampropoulou46 but also TGF-β1 and IL-10, are instrumental in controlling inflammation in experimental models of MS. Reference Arneth47
EBV in MS
Among the infectious factors examined, the B-lymphotropic EBV has been shown to confer increased risk of developing MS, via, as yet, unclear mechanisms. Reference Morandi, Jagessar, ‘t Hart and Gran48 Ninety-six percent of the general population is positive for IgG antibodies against EBV (indicating past infection), while in MS patients this percentage is almost 100%. Reference Guan, Jakimovski, Ramanathan, Weinstock-Guttman and Zivadinov26 Moreover, a prospective cohort study of 955 incident MS patients showed a 97% of EBV (but not other viruses) seroconversion before development of the disease, significantly higher compared to controls. Reference Kjetil, Marianna and H.B.49 Studies and experiments have shaped four major theories about EBV’s role in the pathogenesis of MS: the cross-reactivity hypothesis, Reference Lang, Jacobsen and Ikemizu50 the bystander damage hypothesis, Reference Angelini, Serafini and Piras51 the αβ-crystallin hypothesis, Reference Pender and Burrows52 and the EBV-infected autoreactive B-cell hypothesis. Reference Pender53 Cellular and CSF findings however only partially match the pathophysiology of MS as far as the first three hypotheses are concerned. One important finding is the recent demonstration of molecular mimicry between EBV transcription factor EBNA1 and CNS protein glial cell adhesion molecule (GlialCAM), leading to the production of cross-reactive antibodies with higher affinity towards an intracellular GlialCAM epitope. Reference Lanz, Brewer and Ho54 The fourth hypothesis postulates that EBV-infected autoreactive B cells accumulate in the target organ and orchestrate the disease by producing antibodies and stimulating T cells due to a defect in their elimination by the antiviral CD8+ T cells. Moreover, the EBV antiapoptotic protein BHRF1, produced by both latently and lytically infected cells, inhibits B-cell apoptosis, Reference McCarthy, Hazlewood, Huen, Rickinson and Williams55 resulting in immortalization of the autoreactive B cells it infects. In support of this hypothesis, substantial EBV persistence in B and plasma cells as well as meningeal B-cell lymphoid follicles of all MS cases examined was reported, Reference Serafini, Rosicarelli and Franciotta56 but could not be reproduced in multiple independent replication studies. Reference Sargsyan, Shearer and Ritchie57–Reference Torkildsen, Stansberg and Angelskår60 Overall, the epidemiological associations remain; however, no underlying biological mechanism has been consequently supported by experimental data.
Anti-B-cell Agents as a Therapeutic Strategy
Most anti-B-cell agents are monoclonal antibodies (mAbs); however, small molecules have also emerged as promising agents and have better CNS penetrance (Table 1). B-cell-depleting antibodies can be categorized in 1st-, 2nd-, or 3rd-generation. 1st-generation monoclonal antibodies (mAbs) can be either fully murine (suffix: -omab) or chimeric (65% human, suffix: -ximab), while 2nd-generation ones can be humanized (>90% human, suffix: -zumab) or even fully human (suffix: -mumab). 3rd-generation mAbs consist of a modified Fc region, chimeric, or humanized. Immunogenicity in theory ranges from higher in 1st-generation mAbs to lower in 2nd- and 3rd-generation ones. Reference Whittam, Tallantyre and Jolles61
Table 1: A summary of medicines targeting B cells that have been used in MS

Anti-CD20 mAbs
CD20 is a 33-37kDa transmembrane protein, which spans the membrane four times, thus consisting of two extracellular loops and intracellular C- and N-termini. Although some T cells with CD20 surface expression can be found in all lymphatic organs, are often CD8-positive, can be myelin-specific, Reference Schuh, Berer and Mulazzani62–Reference Sabatino, Wilson, Calabresi, Hauser, Schneck and Zamvil64 and may correlate positively with disease severity, Reference von Essen, Ammitzbøll and Hansen65 CD20 serves a more important role on B cells. The molecule is not expressed throughout the entirety of the B-cell line of differentiation, but only in pre-B cells and mature B cells, with stem cells and the majority of antibody-secreting cells being CD20-negative (Figure 1). Reference Payandeh, Bahrami and Hoseinpoor66–Reference Tedder, Streuli, Schlossman and Saito68 Physiologically, CD20 plays a key role as regulator of calcium influx in the signaling pathways that lead to B-cell differentiation into antibody-secreting plasma cells Reference Santos and Lima69 and its presence on the surface of most, but not all, B cells makes it an attractive target for monoclonal antibody-based therapy. B cells targeted by anti-CD20 monoclonal antibodies are eliminated via three main mechanisms: programmed cell death / apoptosis, complement-dependent cytotoxicity (CDC), or antibody-dependent cellular cytotoxicity (ADCC) processes. Reference Maloney70 Evidence from animal studies shows that anti-CD20 antibody-mediated B-cell depletion may be incomplete in lymph node germinal centers. Reference Schroder, Azimzadeh, Wu, Price, Atkinson and Pierson71 Moreover, cerebrospinal fluid B cells seem to be less affected than peripheral B cells by intravenous rituximab (the first anti-CD20 monoclonal antibody) administration, although the drug itself can be detected in a very low concentration (up to 1000 times lower than in the periphery) behind the BBB. Reference Piccio, Naismith and Trinkaus72–Reference Monson, Cravens, Frohman, Hawker and Racke75 Of note, the limited access of anti-CD20 mAbs to the CNS due to their relatively high molecular weight could, at least to some extent, be overcome with intrathecal (IT) administration of anti-CD20 mAbs. The four main antibodies evaluated for anti-CD20 MS therapy are analyzed below.
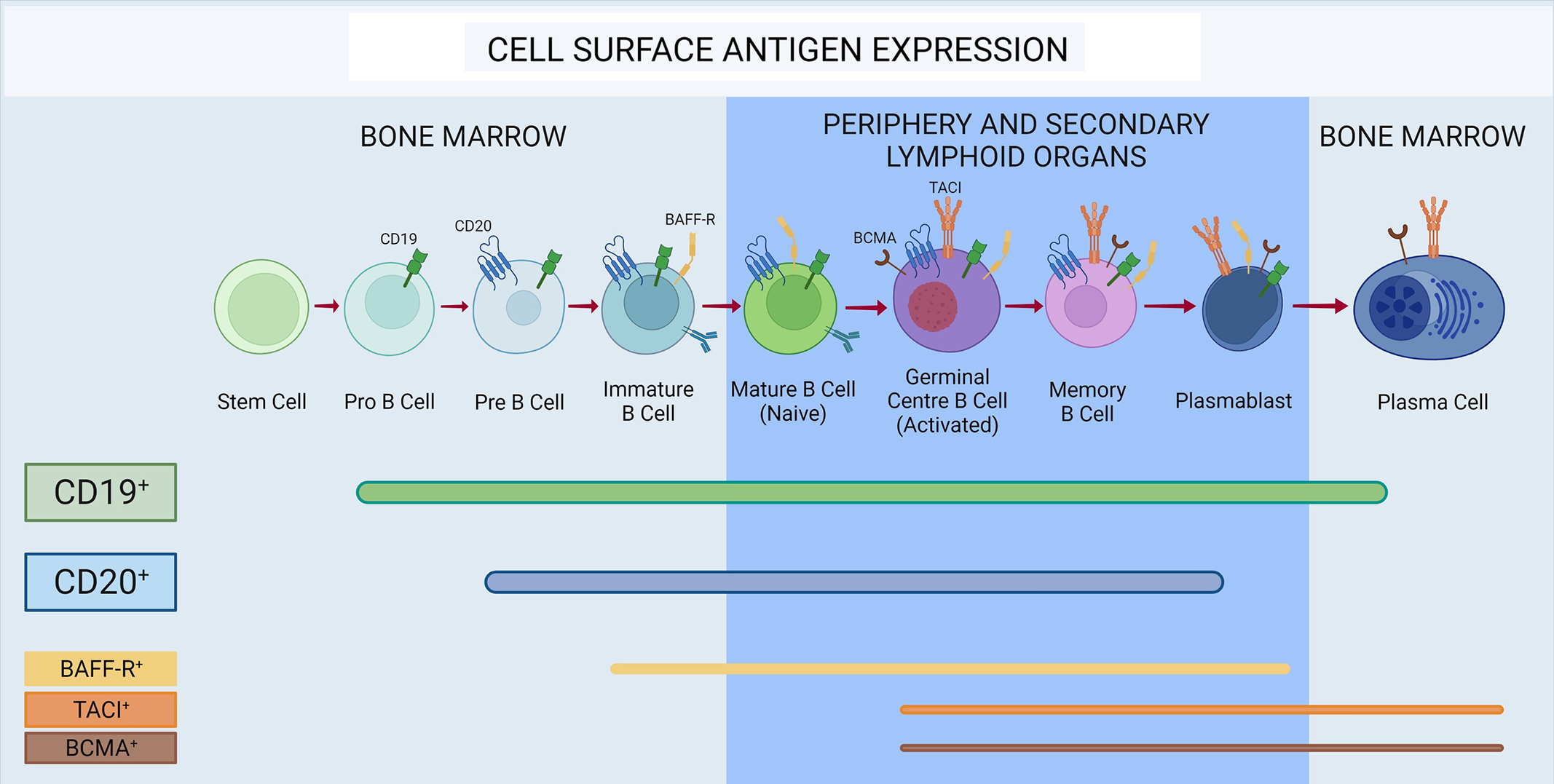
Figure 1: Expression of cell surface antigens throughout B-cell maturation. CD19 is expressed in all stages of B-cell development, with the exception of stem cells and the majority of plasma cells. CD20 is not present on plasma cells, most plasmablasts, pro-B cells, and stem cells. BAFF-receptor (BAFF-R) is expressed on both immature and mature B cells in the germinal center, as well as memory B cells and late plasmablasts. Transmembrane activator and CAML interactor (TACI) and B-cell maturation antigen (BCMA) are expressed on germinal center B cells, memory cells, and antibody-secreting cells.
Rituximab
Rituximab is a 1st-generation chimeric monoclonal antibody (IgG1), engineered by fusing a murine Fab with a human Fc domain. Reference Whittam, Tallantyre and Jolles61 Its elimination half-time is estimated at around 20 days; Reference Bar-Or, Calabresi and Arnold76 it may, however, vary according to sex, body weight, and renal function. Reference Ng, Bruno, Combs and Davies77 Rituximab depletes B cells via ADCC and CDC and has been found to be extremely effective in patients with RRMS. A landmark 48-week, phase 2, double-blind, placebo-controlled study convincingly highlighted the efficacy of rituximab monotherapy in reducing gadolinium-enhanced lesions in patients with RRMS (n = 104). Reference Hauser, Arnold and Vollmer78 In addition, a retrospective observational study of 808 patients with RRMS revealed absence of rebound disease activity upon rituximab cessation, Reference Juto, Fink, al Nimer and Piehl79 whereas rebound activity has been reported with natalizumab Reference lo Re, Capobianco and Ragonese80 and fingolimod cessation. Reference Hatcher, Waubant, Nourbakhsh, Crabtree-Hartman and Graves81,Reference Sacco, Emming, Gobbi, Zecca and Monticelli82
In regard to progressive forms, a phase 3 (n = 439 PPMS patients), double-blind and placebo-controlled trial concluded in 2009 that CD20+ B-cell depletion can slow disease progression in a subgroup of younger patients with PPMS, particularly those with inflammatory lesions (GdELs), as rituximab-treated patients had less increase in T2 lesions and confirmed disease progression was delayed in the subgroup with GdELs. Reference Hawker, O’Connor and Freedman83 Overall, however, the study was negative. Moreover, a large observational, retrospective study from the Swedish MS registry included 822 patients (557 RRMS, 198 SPMS, and 67 PPMS) and confirmed both rituximab’s safety as well as its efficacy in reducing GdELs; GdELs went from 26.2% (pretreatment) to 4.6% in the pooled post-treatment cohort. Reference Salzer, Svenningsson and Alping84 Interestingly, disability remained constant in RRMS patients but increased in SPMS and more so in PPMS patients. The question of whether disability progression differs in treated and untreated patients was tackled by a retrospective cohort study of 88 SPMS patients. This study resulted in significantly lower Expanded Disability Status Scale scores (p < 0.001) and delayed disease progression (p = 0.02) in the rituximab-treated group in comparison to the matched control group. Reference Naegelin, Naegelin and von Felten85 It should be noted however that the rituximab-treated group included more patients with radiologic activity, which may have driven the difference between the two groups.
In clinical practice, rituximab is widely used as an off-label treatment for the management of RRMS, as well as active SPMS, as its safety profile is acceptable, well-characterized, Reference Luna, Alping and Burman86,Reference Alping, Askling and Burman87 and the efficacy evident, despite the lack of phase III trials. Reference Yamout, El-Ayoubi, Nicolas, Kouzi, Khoury and Zeineddine88 The drug is generally well-tolerated by patients all throughout the MS type spectrum, and the main adverse effects are mild to moderate infusion-related reactions (IRRs), typically with the first dose, as well as mild to moderate infections. No cases of progressive multifocal leukoencephalopathy (PML) due to John Cunningham virus, which is mostly seen with natalizumab treatment, Reference Ghajarzadeh, Azimi, Valizadeh, Sahraian and Mohammadifar89,Reference Erickson and Garcea90 have been recorded in MS patients treated with rituximab, and the frequency of PML in non-neurologic patients treated with rituximab seems to range around 1:4000; however, usually these patients have received multiple immunosuppressants. Reference Clifford, Ances and Costello91,Reference Kapoor, Mahadeshwar and Hui-Yuen92 Finally, an added advantage of rituximab is its relatively low cost (biosimilars are also available); however, its off-label prescription is complex and time-consuming for physicians. While open questions remain about optimal dosing and frequency strategies, a common tactic is 2 × 500 or 1000 mg, in a 14-day period, and repeat dosing of 500–1000 mg every 6 months or yearly. Reference Whittam, Tallantyre and Jolles61
Ocrelizumab
Ocrelizumab, an IgG1 immunoglobulin, is a 2nd-generation recombinant humanized anti-CD20 mAb. Reference Syed93 The drug has a terminal elimination half-time of around 26 days, which is not affected by mild renal or hepatic impairment. 94 Compared to rituximab, ocrelizumab mobilizes in vitro lower CDC, but higher ADCC action Reference Klein, Lammens and Schäfer95 and as a humanized molecule is expected to be less immunogenic than rituximab with lower titles of neutralizing anti-drug antibodies. Reference Vugmeyster, Beyer and Howell96,Reference Sorensen and Blinkenberg97
In OPERA I (n = 821 patients) and OPERA II (n = 835 patients), two phase 3, double-blind trials published in 2017, ocrelizumab was associated with a lower annualized relapse rate (by 46–47%) and an impressive reduction of the mean number of GdELs (by 94%) over a 96-week time period compared to interferon beta-1a (p < 0.001). The drug effectively depleted CD19 B cells (CD19 B cells serve as index of B-cell count in anti-CD20 treatment) within 2 weeks (which is when CD19 cells were measured). Reference Hauser, Bar-Or and Comi98 ORATORIO, a phase 3, double-blind, placebo-controlled trial, examined ocrelizumab’s efficacy in managing PPMS progression. Results from 732 patients revealed that ocrelizumab was associated with lower rates of clinical and MRI progression than placebo. Because in this study the effect was driven by a fraction of PPMS that had evident MRI inflammation, EMA has approved the drug only in inflammatory PPMS, whereas other agencies such as the FDA and Swissmedic have not applied this restriction. Reference Montalban, Hauser and Kappos99
The most common adverse effects of ocrelizumab include mild and manageable IRRs, like pruritus, rashes, throat irritations, and flushing, but their severity and frequency decrease with the number of infusions. Generally, mild to moderate infections occur in 30% of patients, but severe ones are relatively rare. Other adverse events such as extremity pain, diarrhea, and peripheral edema may also occur in rare cases. Reference Rommer and Zettl100,Reference Rommer, Dudesek, Stüve and Zettl101
Ocrelizumab is administered intravenously according to a fixed dosing schedule, as approved based on the phase 3 studies. An initial dose of 600 mg is divided in 2 × 300 mg with a 2-week time interval. Subsequent doses of 600 mg are given in a single infusion once every 6 months. 94 Interestingly, a post hoc analysis from ORATORIO, where patients with lower body weight (and respectively higher ocrelizumab dose per kg) suffered less progression of deficits, prompted a currently ongoing clinical trial that examines the safety and efficacy of higher than standard ocrelizumab doses (1200 mg for body weights <75 kg, or 1800 mg for body weights >75 kg) in PPMS. Reference Gibiansky, Petry and Mercier102,103
Ofatumumab
Ofatumumab is a 2nd-generation, fully human IgG1 mAb Reference Florou, Katsara, Feehan, Dardiotis and Apostolopoulos104 that depletes circulating CD20 B cells via ADCC Reference Masoud, McAdoo, Bedi, Cairns and Lightstone105 and CDC. 106 Two identically designed, double-blind, phase 3 clinical trials, ASCLEPIOS I and II, compared the efficacy of subcutaneously administered ofatumumab to that of oral teriflunomide, the oral pyrimidine synthesis inhibitor. The trials enrolled 1882 patients in total in 1.6 years, and their results indicated a statistically significant advantage of ofatumumab over teriflunomide in suppressing both new relapses and GdEL activity (the latter by 94–97%). Side effects were reported to be mild to moderate and included injection-related reactions, headache, and infections (in 51.6% of patients treated with ofatumumab) such as nasopharyngitis, upper respiratory, and urinary tract infection. Reference Hauser, Bar-Or and Cohen107 Consequently, the FDA approved ofatumumab as a therapy for RRMS, CIS, and active SPMS in the form of an auto-injector pen, while the EMA for relapsing, active MS. 106 Ofatumumab was approved for subcutaneous use at a dose of 20 mg/0.4 mL once per week for the first 3 weeks of treatment and once monthly thereafter. 108
Ublituximab
Ublituximab is a 3rd-generation anti-CD20 glycoengineered chimeric IgG1 mAb that exerts its action primarily via ADCC, which is facilitated by defucosylation of its Fc region and thereby increased affinity for FcγRIIIa. Reference Whittam, Tallantyre and Jolles61 A 48-week, placebo-controlled, phase 2 trial of ublituximab in 45 RRMS patients established that 150 mg iv on day 1 and 450–600 mg on day 15 and week 24 were able to efficiently deplete B cells within 4 weeks (which is when B cells were measured); moreover, 74% of patients achieved no evidence of disease activity status (NEDA), that is had no relapses, no radiological disease activity, and no progression of disability. Similarly to the other CD20 agents, adverse effects comprised mild to moderate IRRs and upper respiratory infections, influenza, nasopharyngitis, sinusitis, and fungal infections. Reference Fox, Lovett-Racke and Gormley109 In follow-up, two double-blind, phase 3 trials [ULTIMATE I (NCT03277261) and II (NCT03277248)] will assess ublituximab’s efficacy and safety compared to teriflunomide in 880 patients with RRMS. Reference Mealy and Levy110,111
Anti-CD19 mAbs
CD19 belongs to the Ig superfamily and along with CD21, CD82, and CD225 contributes to the formation of a multimolecular signal-transduction complex that ultimately leads to the activation of PI-3 kinase. Reference Tedder112 Compared to CD20, CD19 is expressed on B cells of earlier developmental stages as well as in more antibody-secreting cells and is thus an appealing therapeutic target (Figure 1). Reference Chen, Gallagher, Monson, Herbst and Wang113 In addition to having a broader expression during B-cell stages of development and differentiation, CD19, unlike CD20, is selectively expressed on B cells and not T cells. Reference Schuh, Berer and Mulazzani62 A phase 1 study assessing the pharmacokinetic (intravenous and subcutaneous) profile of inebilizumab, a humanized afucosylated IgG1κ anti-CD19 mAb, Reference Herbst, Wang and Gallagher114 has been conducted in patients with relapsing MS with positive results, 115 but no phase III trials for MS are currently known to be underway.
Atacicept
Atacicept is a human recombinant fusion protein, consisting of a human IgG Fc portion and the extracellular domain of TACI receptor that binds both BAFF and a proliferation-inducing ligand (APRIL). Reference Magliozzi, Marastoni and Calabrese116 The drug therefore competes for BAFF and APRIL binding with native TACI, which is both membrane-bound and soluble, Reference Hoffmann, Kuhn and Laurent117 as well as, to a lesser extent, with the other receptors of the BAFF-APRIL system (BAFF-R and BCMA). Reference Benson, Dillon and Castigli118,Reference Hartung and Kieseier119 After improving rheumatoid arthritis and systemic lupus erythematosus (SLE), Reference van Vollenhoven, Kinnman, Vincent, Wax and Bathon120 atacicept was tried in MS.
Subcutaneous atacicept was evaluated in a 36-week, phase 2, double-blind, and placebo-controlled trial in 255 patients with relapsing MS. The trial was prematurely terminated when an increase in inflammatory disease activity was noticed despite immunoglobulin and naïve B-cell decrease, which led to the suspension of every atacicept trial in MS. Reference Hartung and Kieseier119,Reference Kappos, Hartung and Freedman121,122 Another 36-week, phase 2, double-blind, and placebo-controlled atacicept trial in 34 patients with unilateral optic neuritis as clinical isolated syndrome also showed disease exacerbation, with a significantly higher proportion of patients converting to clinically definite MS compared with placebo. Reference Sergott, Bennett and Rieckmann123 As atacicept effectively depletes naive B cells and induces a transient but marked increase in memory B cells (especially class-switched ones), Reference Hoffmann, Kuhn and Laurent117,Reference Lühder and Gold124,Reference Jelcic, al Nimer and Wang125 possible reasons why atacicept aggravated MS include elimination of regulatory naïve B cells and enhancement of pathogenic memory B-cell function. Reference Baker, Pryce, James, Schmierer and Giovannoni126,Reference Baker, Marta, Pryce, Giovannoni and Schmierer127
Belimumab
Belimumab is a human IgG1λ recombinant monoclonal antibody directed against BAFF that prevents BAFF from interacting with its three receptors on the surface of B cells, thereby reducing B-cell survival, differentiation, and antibody production. Reference Halpern, Lappin and Zanardi128,Reference Dubey, Handu, Dubey, Sharma, Sharma and Ahmed129 Interestingly, belimumab administration does not result in overt immunosuppression. 130 While being moderately effective and FDA-approved for the treatment of SLE since 2011, it failed in myasthenia gravis, Reference Hewett, Sanders and Grove131 a disease mediated by pathogenic autoantibodies. Reference Stohl and Hilbert132 A phase 2, open-label trial of subcutaneous belimumab in addition to ocrelizumab (standard dose) in 40 patients with RRMS was scheduled to start within 2021. 130
Evobrutinib
Evobrutinib is a small molecule drug that binds permanently to and deactivates Bruton’s Tyrosine Kinase (BTK). BTK is an integral part of the BCR signaling cascade that affects B-cell activation and is essential for B-cell maturation and their ultimate, terminal differentiation into memory or plasma cells. Of interest, BTK is involved in the entry of B cells into follicular structures. Knockout or absence of BTK results in lack of B-cell activation, moreover almost complete lack of peripheral B and plasma cells and low circulating immunoglobulin. Reference Dingjan, Middendorp, Dahlenborg, Maas, Grosveld and Hendriks133–Reference Torke and Weber136 Importantly, about 75% of the CNS cells that express BTK are microglial, while BTK expression levels in the brain increase after demyelination. Reference Martin, Aigrot and Grenningloh137 As evobrutinib can bypass the BBB and enter the CNS, it can affect microglial cells and B cells within the CNS.
Evobrutinib was the first BTK inhibitor (BTKI) to be tested as a monotherapy in relapsing MS. Reference Becker, Martin and Mitchell138 In a double-blind, phase II trial (n = 267), evobrutinib was tested against placebo and dimethyl fumarate. The results showed that patients who received 75 mg of daily evobrutinib had significantly fewer GdELs during weeks 12 through 24 than those who received placebo (1.69 ± 4.69 against 3.85 ± 5.44, p = 0.005), while adverse effects were minimal (e.g. nasopharyngitis, alanine aminotransferase, and aspartate aminotransferase level elevation). 139,Reference Montalban, Arnold and Weber140 Evobrutinib is now being advanced to phase III evaluation, along with several other BTKI (some of them with reversible BTK binding); fenebrutinib, ibrutinib, and tolebrutinib. 141
While CD19/20 B-cell depletion has shown tremendous efficacy in reducing clinical and radiological MS activity, it raises several safety concerns on humoral deficiency with long-term usage in addition to a reduced response to vaccination. Reference Luna, Alping and Burman86,Reference Nazi, Kelton and Larché142,Reference Bar-Or, Calkwood and Chognot143 These disadvantages could possibly be avoided with inhibition of B-cell activation and maturation with small molecules such as BTKIs. Reference Torke and Weber136,Reference Dolgin144 In contrast with antibody-based B-cell depletion, BTKIs do not destroy or lastingly minimize the frequency of peripheral B cells, but seem to prevent the development of pathogenic B cells. Reference Torke, Pretzsch and Häusler145 Their effect on disease activity does not seem to be as impressive as that of anti-B-cell antibodies, and they cannot control the pathogenic properties of B cells as rapidly; however, they are smaller in size, can penetrate the CNS, target microglia, and might therefore have a better effect on disability progression. Reference Boschert, Crandall and Pereira146
B-Cell-Targeted Therapies and Pregnancy
As MS largely affects female patients with childbearing potential, the utilization of B-cell-targeted therapies in women of childbearing age deserves special mention. Reference Tisovic and Amezcua147,Reference Wallin, Culpepper and Campbell148 Rituximab-associated B-cell depletion persists long after the drug’s elimination, which occurs approximately 3 months after the last infusion. Thus, conception can be considered safe 3 months after the last infusion without significant risk of fetal exposure. But even if a woman conceives before rituximab’s effective elimination, IgG1 subclass mAbs cannot cross the placenta barrier during the first trimester, resulting in low chance of fetal exposure. Reference Das, Damotte and Gelfand149 Importantly, rituximab administration and concurrent B-cell depletion have not been linked to increased risk of adverse pregnancy outcomes compared with the expected incidence in population. Reference Smith, Hellwig, Fink, Lyell, Piehl and Langer-Gould150 Also, infants breastfed under anti-CD20 treatment had normal B-cell counts, and no negative impact on health and development was attributed to breastfeeding in the 1-year follow-up period. Reference Ciplea, Langer-Gould and de Vries151 Although data regarding ocrelizumab administration in this population group are limited, it is reasonable to apply the same principles as with rituximab. One additional advantage of CD20 depletion in terms of family planning is that discontinuation of therapy is not associated with a rebound phenomenon, as has been observed with natalizumab. In that regard, a cohort study regarding the safety of anti-C20 mAbs rituximab and ocrelizumab during the last 12 months before or during pregnancy concluded that the drugs are effective in controlling disease in women with RRMS, during and partly after pregnancy. However, B-cell monitoring is essential both for the newborn and for the mother after delivery, and larger studies are required to assess their safety profile and to establish the best time to restart the therapy after delivery. Reference Kümpfel, Thiel and Meinl152 Recent recommendations suggest prioritization of MS management and conception postponement in cases of highly active MS and contraception for up to 4 months after ocrelizumab administration.Reference Wiendl, Gold and Berger 153
Conclusion
The therapeutic criterion underlines that B cells not only participate in the pathogenesis of MS but can act as the orchestrators of the inflammatory processes. As shown by clinical trials and real-world data, B-cell-targeting agents (in particular CD20-depleting agents) have established a new era in MS therapeutics and immunotherapy in general, considering their remarkable efficacy and safety profile. Long-term safety, especially increased risk of infection with slowly but gradually decreasing total serum immunoglobulin levels, remains a significant concern that has a limiting effect on anti-CD20 usage in clinical practice. Regular monitoring of immunoglobulin levels (e.g. before each follow-up infusion) can help timely detection of a decrease and lowers the risk of infection due to associated immunodeficiency. Reference Keystone, Fleischmann and Emery154–Reference van Vollenhoven, Emery and Bingham156 Future studies will further inform on long-term effects of CD20-targeting medications, on the use of oral BTKI agents and determine the new therapeutic algorithm that will likely move more towards induction rather than escalation.
Acknowledgements
Figure was created with BioRender.com. The publication of the article in OA mode was financially supported by HEAL-Link.
Funding
PK, IS, and LS received no funding in relation to the present topic. PS is supported by the Onassis Foundation.
Conflict of Interest
PK and IS declare no conflicts of interest. LS is the site investigator in the trials MUSETTE (BN42082) and GAVOTTE (BN42083), sponsored by F. Hoffmann La-Roche Ltd. PS has received a travel grant from Sanofi and research funding by the Onassis Foundation.
Statement of Authorship
Conceptualization: PS.
Drafting: PS, PK, IS.
Editing: PS, PK, IS, LS.




