We report a 37-year-old obese (BMI 39 kg/m2) woman who presented with a 6-month history of progressive bifrontal headaches and bilateral pulsatile tinnitus followed by new-onset bilateral blurry vision. Exam showed normal pupillary response, extraocular movements, and visual acuity. Fundoscopy revealed bilateral cotton wool spots and grade 1 optic disc edema. CT head was normal, and lumbar puncture yielded increased opening pressure (26 cm H20) but was otherwise unremarkable. MR of brain/orbits showed partial empty sella, prominent and tortuous optic nerve sheaths, and posterior globe flattening; MR venogram showed focal stenosis at the right transverse–sigmoid sinus junction (Figure 1) and a congenitally hypoplastic left transverse sinus. She was diagnosed with idiopathic intracranial hypertension (IIH) and started on acetazolamide. Treatment was later switched to furosemide and topiramate due to nausea/vomiting and metabolic acidosis despite lowest possible dose. Nine months after diagnosis, headaches persisted and her visual acuity declined from 20/20 bilaterally to 20/80 on left and 20/100 on right with progression to grade 2 disc edema. Right optic nerve sheath fenestration was performed but failed to improve her symptoms. Further investigation with cerebral angiography showed severe stenosis at the right transverse–sigmoid junction (pressure gradient of ~25 mmHg) prompting venous stenting, which improved drainage and relieved the pressure gradient (Figure 2). Postoperatively, she experienced full resolution of her headaches and optic disc edema. At 7-month follow-up, she had only pulsatile tinnitus and mild intermittent visual disturbances despite restored visual acuity (20/25 on left and 20/20 on right).
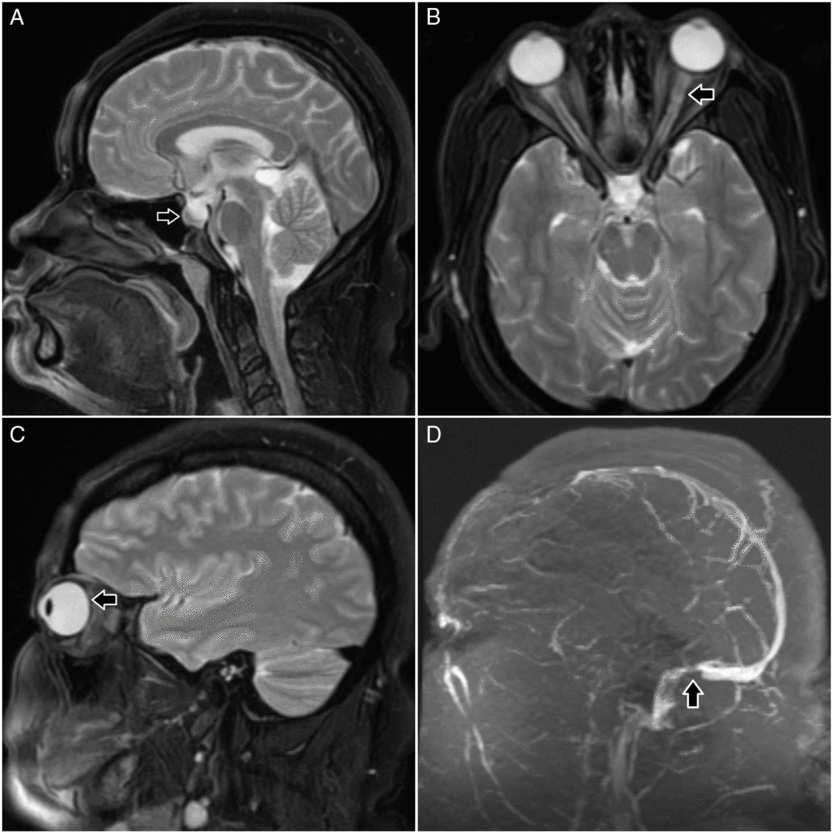
Figure 1: Brain and orbits MRI (A–C). Sagittal T2 (A) shows partial empty sella; axial (B) and sagittal (C) T2 show prominent and tortuous optic nerve sheaths and flattening of the posterior globes, respectively. Brain MR venogram (D) shows a focal stenosis at the junction of the sigmoid and transverse sinus.
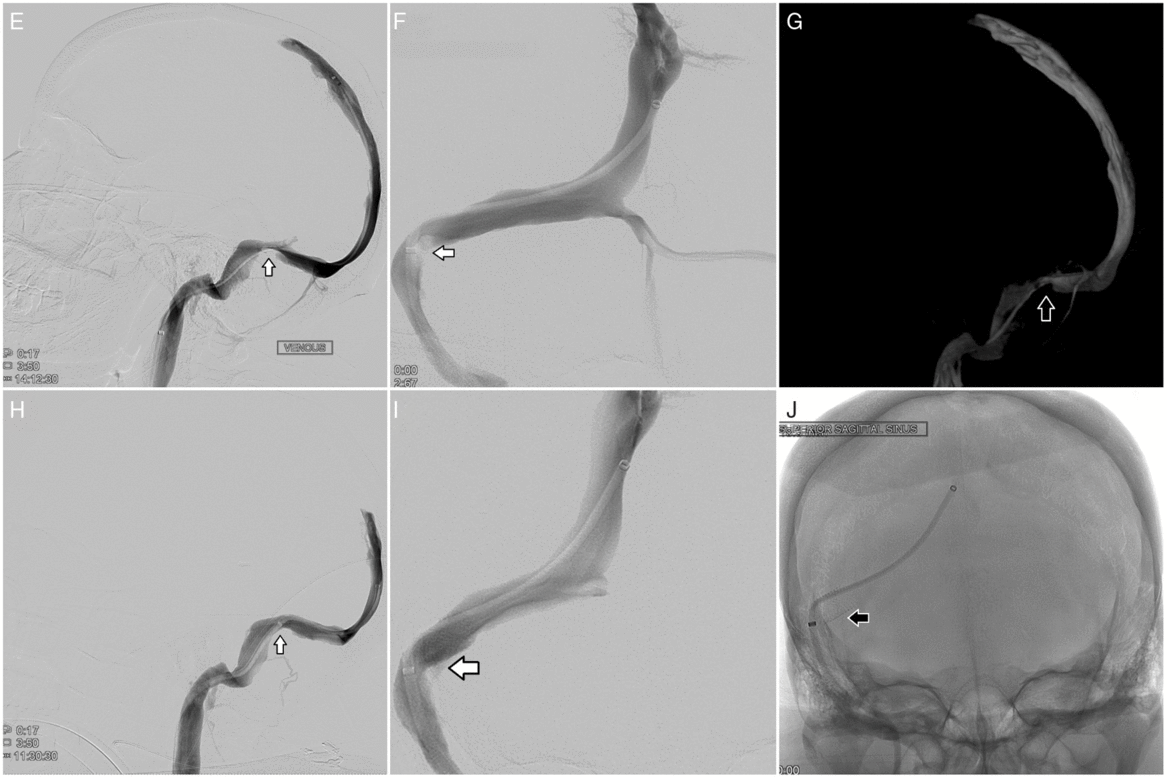
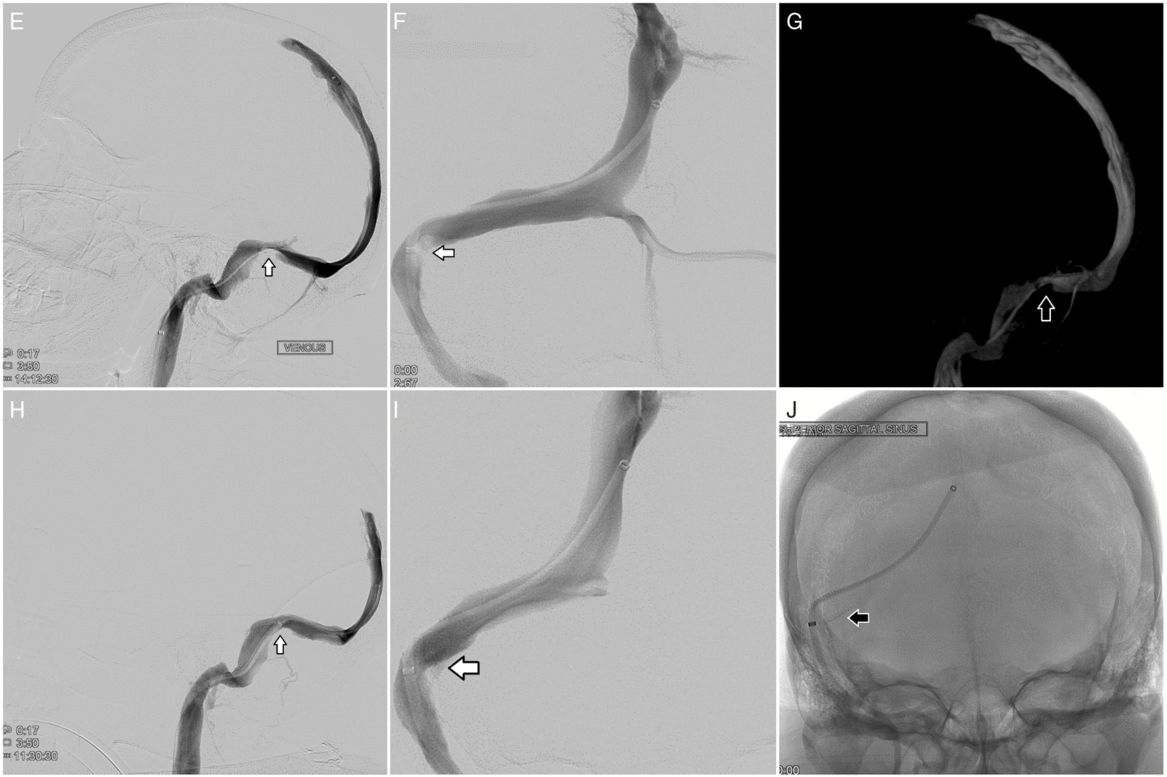
Figure 2: Cerebral angiography (E–J). Pre-procedural cerebral angiography shows severe stenosis of the confluence of the right sigmoid and transverse sinus with a 25-mmHg pressure gradient: lateral view (E), anterior–posterior view (F), and 3D reconstruction from cone beam CT (G). Post-procedural cerebral angiography following placement of a Precise Pro RX carotid stent (8 x 40 mm) across the stenotic segment of the right sigmoid and transverse sinus shows resolution of stenosis, improved venous drainage, and resolution of gradient pressure: lateral view (H) and anterior–posterior view (I and J).
IIH is characterized by elevated intracranial pressure without evidence of mass lesion or ventriculomegaly. It is classically associated with obese women of childbearing age and typically presents with headaches, papilledema, pulsatile tinnitus, and visual disturbances that can progress to blindness.Reference Shields, Shields, Yao, Plato, Zhang and Dashti1 IIH occurs with an incidence of 1–2 per 100,000 individuals in North America, and 14.9–19.3 per 100,000 among obese women aged 20–44.Reference Fargen, Liu, Garner, Greeneway, Wolfe and Crowley2 Diagnostic criteria include symptoms of increased intracranial pressure, normal neurological exam excluding possible visual deficits, negative brain imaging via CT/MRI, and opening pressure >25 cm H20 on lumbar puncture (LP).Reference Shields, Shields, Yao, Plato, Zhang and Dashti1 While pathophysiology remains controversial, recent studies have established the presence of unilateral or bilateral transverse sinus stenosis in most IIH patients, representing the most reliable imaging indicator of the disease state.Reference Satti, Leishangthem, Spiotta and Chaudry3, Reference Morris, Black, Port and Campeau4 CT or MR venography and cerebral angiography can identify obstruction in the setting of IIH, but detection of significant pressure gradients via venous manometry is a more sensitive method.Reference West, Greeneway and Garner5
Due to the role of sinus stenosis in the etiology of IIH, dural venous sinus stenting (DVSS) has emerged as a promising therapeutic option.Reference Higgins, Cousins, Owler, Sarkies and Pickard6 While no standardized guidelines currently exist, traditional treatment strategies include weight loss, serial LPs, and carbonic anhydrase inhibitors.Reference Shields, Shields, Yao, Plato, Zhang and Dashti1 Cerebrospinal fluid diversion via shunt placement and optic nerve sheath fenestration are also used in refractory patients but carry significant complication and recurrence rates.Reference Fargen, Liu, Garner, Greeneway, Wolfe and Crowley2 A retrospective meta-analysis of 410 IIH patients undergoing DVSS reported significant post-procedure improvement in headache (82%), papilledema (92%), and visual acuity (78%).Reference Leishangthem, SirDeshpande, Dua and Satti7 The overall complication rate was 4.9%, and major complications comprising intracranial hemorrhage, subarachnoid hemorrhage, and subdural hematoma were seen in 1.5% of patients.Reference Leishangthem, SirDeshpande, Dua and Satti7 Significant symptoms recur in <10% of patients.Reference Nicholson, Brinjikji and Radovanovic8 Selection criteria remain ambiguous, but DVSS should be considered in medically refractory IIH patients with hemodynamically significant dural sinus stenosis and pressure gradient ≥8 mm Hg.Reference Satti, Leishangthem, Spiotta and Chaudry3
Management of IIH requires a multidisciplinary approach spanning primary care, neurology, radiology, ophthalmology, and neurosurgery. This report highlights the importance of diagnostic evaluation for venous sinus stenosis in the setting of intractable IIH and adds to the growing body of evidence that DVSS is a safe, effective technique for long-term relief.
Disclosures
All authors report no disclosures.
Statement Of Authorship
RC: chart and literature review, drafting manuscript. FAN: content design, drafting and revising manuscript. PJD: case report supervision, revising manuscript. SRC: case report supervision, revising manuscript. KRN: case report supervision, revising manuscript.