Infection is a well-documented complication of cranioplasty, occurring in 3.7-16.7% of cases.Reference Chang, Hartzfeld, Langlois, Mahmood and Seyfried 1 - Reference Vahtsevanos, Triaridis and Patrikidou 8 As delayed recognition can potentially result in significant morbidity and mortality, early detection of these post-operative episodes is crucial. Leakage from the incision site, swelling of tissue overlying the flap, and exposed hardware are all common presenting signs suggesting the presence of localized infection following this procedure. Systemic signs, however, are not as readily distinguishable, making their use in the diagnosis and management of this complication uncertain.
Systemic evidence of the early stages of infection can be summarized in general terms using the features of the Systemic Inflammatory Response Syndrome (SIRS), where diagnosis requires the presence of two of four signs: fever, leukocytosis, tachypnea, and tachycardia. 9 Studies looking specifically at the role of elements from this syndrome in central nervous system infections are scarce but existing evidence does lean towards a greater utility in acute, more so than chronic, infectious processesReference Vates, Berger and Wilson 10 , Reference Candon and Frerebea 11 The utility of SIRS elements in post-cranioplasty infection, however, has not been directly addressed in the literature. Therefore, in this study, we focused on two commonly recorded signs in patients presenting with suspected infection after cranioplasty, fever and leukocytosis, in order to determine their ability to predict subsequently confirmed infection. In addition, we sought to identify any factors that may limit their applicability.
Methods
After obtaining Institutional Review Board approval, a retrospective review was conducted to identify all patients who had undergone cranioplasty at the Massachusetts General Hospital from 2001 to 2011. Of the 239 cases identified, 27 underwent further surgery for suspicion of post-operative infection. Infection was defined either by evidence of frank purulence as described in operative records and/or the presence of positive intra-operative cultures from a non-superficial source, such as subcutaneous tissue, bone, or epidural, subdural, and/or intracranial collections.
In addition to characterizing factors such as age and gender, the following data was collected from patient records: The indication for and type of craniectomy performed; the method of bone flap preservation, if applicable; the use of hardware during treatment such as a shunt or extra-ventricular drain; and the type of bone flap implanted during cranioplasty. In regard to infectious documentation, the following data was collected on the date of infectious presentation after cranioplasty: The white blood cell (WBC) count and forehead temperature as measured using a temporal thermometer; the presence of exposed hardware or bone; and the presence of wound leakage. Further information collected included blood culture and non-superficial intra-operative culture results. Although pain at the site is classically described with post-operative infections, pain scores were not recorded or analyzed, as the data was considered to be too subjective.
The type of craniectomy was classified as convexity or bifrontal, while the indication for craniectomy was classified as either trauma or stroke. Bone flap preservation, when applicable, was classified as in vivo or frozen, while the type of bone flap implanted was classified as either autologous or synthetic. Infectious presentation was defined as the date the patient presented to hospital with infectious symptoms. On the same date, blood was drawn for WBC count and culture, temperature was measured to assess for fever and the incision was inspected for exposed hardware or bone and leakage. An elevated WBC count was defined as ≥11.0×109/L and fever was defined as a temporal temperature ≥101.3oF (≥38.5oC), which are the cutoffs used at this center.
Statistical analysis was performed using the R programming environment (Vienna, Austria). A p value of less than 0.05 was pre-defined to indicate a statistically significant result.
Results
A total of 239 cranioplasty patients were identified, with 27 (11.3%) of these undergoing repeat surgeries for the treatment of infection. Infection was suspected based on a combination of clinical and radiographic factors, including swelling around the incision site, wound drainage or breakdown, leukocytosis, and/or fluid collections on computed tomography or magnetic resonance images (MRI). Infection was diagnosed in all suspected cases and was defined either by positive intra-operative cultures and/or frank purulence reported in the operative records. In all cases, the bone flap was removed and discarded and intravenous antibiotics were given for a minimum of six weeks treatment duration.
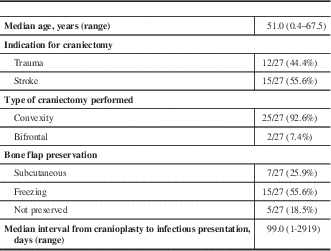
Of the patients that developed infection, the median age at time of craniectomy was 51.0 (range 0.4-67.5), and 63.0% (17/27) of patients were male. Indications for craniectomy included trauma in 44.4% (12/27) of patients, and stroke for the remainder (15/27; 55.6%). Of the craniectomies performed, 92.6% (25/27) consisted of unilateral convexity bone removal and the remaining 7.4% (2/27) were bifrontal craniectomies. The bone flap was preserved in 22 of the 27 cases either in vivo by abdominal subcutaneous implantation (7/27, 25.9%), or by freezing (15/27, 55.6%). In the 18.5% (5/27) of cases where it was not preserved, a synthetic bone flap was inserted for reconstruction of the skull defect. The median interval from the date of cranioplasty to the date of presentation with infectious symptoms was 99 days, with values ranging from 1 to 2919 days. A summary of clinical parameters can be found in Table 1.
Table 1 Clinical parameters
At presentation to hospital for infectious symptoms, 14.8% of patients (4/27) had a WBC count ≥11.0×109/L, and 7.4% (2/27) had a temporal temperature ≥101.3oF. In terms of the appearance of the incision, 18.5% of patients (5/27) had exposed hardware or bone visible through the incision, and 51.9% (14/27) had drainage from the wound. Blood cultures were drawn in 26 of the 27 patients and all returned no growth. Non-superficial intracranial cultures, meaning from subcutaneous tissues or deeper, were collected during surgery in 25 of the 27 patients, with 22 of these (81.5%) yielding positive cultures. Seven organisms were isolated in total, with the tissue collected from some patients growing more than one organism. The most frequently encountered organism was Propionibacterium acnes. A summary of the infectious characteristics of the studied patients can be found in Table 2.
Table 2 Details on infections
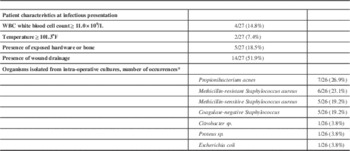
* Some operations yielded more than one organism
Of the 27 patients with infection, 8 had intra-axial collections, 18 had subdural collections, and 1 patient had an epidural collection. Of the patients who had either leukocytosis (four patients) or fever (two patients) at presentation, none had intra-axial collections, five had subdural collections, and one had an epidural collection. Fourteen of 27 patients (51.9%) underwent MRI with diffusion weighting, with seven of these (50%) showing restricted diffusion at the infected site. Overall median follow-up time was 29.7 months (range 1.7-122.7).
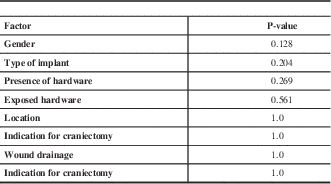
In this series, the false-negative rate was 92.6% for fever and 85.2% for leukocytosis. Univariate logistic regression analysis revealed no significant predictors of false-negative outcomes for these two variables (Table 3).
Table 3 Predictors of false-negative leukocytosis on presentation by univariate analysis
Discussion
Similar to the diagnosis of infection in other post-surgical patients, fever and leukocytosis are commonly available tests utilized by the neurosurgeon in the investigation of post-cranioplasty infection. We have shown, however, that the utility of a high fever or elevated WBC count can be deceiving, in that they carry a false-negative rate of 92.6% and 85.2%, respectively. Unfortunately, a small sample size prevented the finding of any significant factors that may predict outcomes based on these variables.
This lack of systemic signs such as leukocytosis and fever in the presentation of post-cranioplasty infection mirrors the relative lack of these findings in other central nervous system infections. VatesReference Vates, Berger and Wilson 10 reported on 24 cases of intra-operatively diagnosed pituitary abscess, where less than one third of patients presented with systemic signs and symptoms and most presented with findings related to mass effect. Another example is that of Candon,Reference Candon and Frerebea 11 who reviewed 73 cases of intramedullary spinal cord abscesses. The authors found that patients who presented with less than one week of symptoms, the ‘acute-onset group’, were more likely to have fever and/or leukocytosis, as compared to the ‘chronic group’, who presented with greater than six weeks of symptoms. This is similar to our study, in that most patients described an insidious onset of symptoms rather than an acute event, making systemic findings less likely.
Another reason for such a low incidence of fever and leukocytosis in post-cranioplasty infections could be that neurosurgical patients are frequently on medications that may mask infectious signs and symptoms. These include corticosteroids and chemotherapy, the use of which can cause immunosuppression and prevent the body from mounting an appropriate immune response. However, no patients in this series were receiving long-term corticosteroids or chemotherapy. Alternatively, the low rates of systemic response may be related to the pathogenic mechanism involved in the development of the infection. Blunting of the inflammatory response, that is, the recruitment of neutrophils and other plasma components to the surgical site, may occur due to the isolated location of the infection, as purulent fluid collections in these cases are often localized in the form of an abscess.Reference Jovanovic, Illic and Jankovic 12
Other evidence for lack of systemic involvement in cranioplasty infection is that all patients in this study had negative blood cultures, a trend that questions the need for this investigation in this subset of patients. A recent study investigated the general need for blood cultures in patients with suspected bacteremia, concluding that isolated fever or leukocytosis alone are not sufficient to predict bacteremia, while SIRS is a sensitive but non-specific predictorReference Coburn, Morris, Tomlinson and Detsky 13 Our results support this conclusion in that all but one patient met criteria for SIRS, while all patients tested negative for bacteremia. Based on these results, it may be likely that blood cultures are not warranted in cases of post-cranioplasty infection, unless the patient is exhibiting systemic signs of disease such as SIRS or sepsis. Furthermore, specific scoring systems for SIRS and its relation to the underlying etiology may help predict outcomes for those patients in critical care settingsReference Han and Liang 14 , Reference Brun-Bruisson 15 and post-discharge surveillance systems may provide quality assurance in surgical trauma patients.Reference McIntyre, Warner, Nester and Nathens 16
The pre-test probability of any diagnostic test is related to the disease prevalence, therefore knowledge factors that influence infection rates must be taken into consideration for each clinical scenario.Reference Walcott, Redjal and Coumans 17 There have been multiple attempts to identify predictors of infection development following cranioplasty. For one, the timing of cranioplasty relative to the initial craniectomy has been suggested to influence the subsequent development of infection. For those undergoing “delayed” cranioplasty (studies varying from 3-12 months following craniectomy), infection rates were lower.Reference Rish, Dillon and Meirowsky 6 , Reference Thavarajah, De Lacy, Hussien and Sugar 7 , Reference Cheng, Weng, Yang, Lee, Wang and Chang 18 However, one contrasting study demonstrated that waiting a shorter period of time, from zero to six months, resulted in lower infection rates.Reference Chang, Hartzfeld, Langlois, Mahmood and Seyfried 1 A systematic review concluded that there was no statistically significant association between the interval to cranioplasty and subsequent infection.Reference Yadla, Campbell, Chitale, Maltenfort, Jabbour and Sharan 19 Additionally, it has been hypothesized that the type of cranioplasty material and/or storage method may influence infection rates, although the results are conflicting. Inamasu reported that in patients whose craniectomy was subsequent to a traumatic injury, autologous bone flaps that were cryopreserved had higher infection rates after cranioplasty than those stored subcutaneously.Reference Inamasu, Kuramae and Nakatsukasa 4 Another study, by Matsuno, found that frozen autologous and polymethylmethacrylate (PMMA) implants had higher infection rates compared to titanium mesh implants (38.5% and 6.3% vs. 0.Reference Matsuno, Tanaka and Iwamuro 5 Conversely, Chang found that frozen autologous implants were 13.8% less likely to get an infection compared to titanium mesh and PMMA.Reference Chang, Hartzfeld, Langlois, Mahmood and Seyfried 1 Similar to the timing of surgery, a systematic review in 2011 by Yadla found no significant difference between autograft and allograft materialsReference Yadla, Campbell, Chitale, Maltenfort, Jabbour and Sharan 19 Interestingly, four studies investigating the feasibility of re-implanting an infected autograft after meticulous debridement, scrubbing with iodine solution and followed by intravenous antibiotics, showed success in 69-100% of casesReference Chiang, Steelman and Pottinger 2 , Reference Auguste and McDermott 20 - Reference Widdel and Winston 22 Further study is needed to identify predictors of cranioplasty infection, the results of which may also optimize diagnostics.
Of the patients in our study who underwent an MRI scan, 50% had restricted diffusion at the infection site. The only study looking at diffusion weighting in the diagnosis of post-cranioplasty infection reports it to be a useful investigation,Reference Tamaki, Eguchi, Sakamoto and Teramoto 23 however our results are clearly conflicting. Despite being useful in the differentiation of abscesses from tumors,Reference Xu, Li, Yang, Du, Li and Wang 24 restricted diffusion in central nervous system infections is associated with a high false-negative rate, especially when the infection is located extradurally.Reference Farrell, Hoh, Pisculli, Henson, Barker and Curry 25 Although not performed on any of the patients included in this study, white blood cell tagged scans, such as indium-111 or gallium scans, can be helpful in the diagnosis of cranial infection. Considering these nuclear medicine investigations are classically described in the diagnosis of osteomyelitis,Reference Bruni, Padovano, Travascio, Schillaci and Simonetti 26 , Reference Liberatore, Drudi, Tarantino, Prosperi, Fiore and Missori 27 it would be interesting to see if their utility is maintained in infections of bone that is re-inserted into the body, as in the setting of cranioplasty infections.
The main limitations of this study are its small sample size and retrospective nature. To maximize accuracy, the database used to identify patients was confirmed by comparing surgeon records with anesthesia and billing records. Cases of suspected cranioplasty infection not undergoing operative intervention would be useful to analyze, however there was no accurate means of identifying these patients with a retrospective study design. The results do show a convincing trend in the high false-negative rates of fever and leukocytosis in this patient subset. An ongoing, prospectively identified cohort is now established based on these preliminary findings.
Conclusion
The utilization of fever and elevated WBC count in the diagnosis of post-cranioplasty infection is associated with a high false-negative rate, making the absence of these features insufficient to exclude the diagnosis of infection. This information may serve useful for practitioners placing a high importance on these factors, as surgical treatment should be tailored based on the clinical suspicion of infection, rather than systemic signs.
Disclosures
All authors have seen and approved this manuscript and have no disclosures to report. No portion of this work has been previously published or presented. Fady Girgis, Brian Walcott, Churl-Su Kwon, Sameer Sheth, Wael Assad, Brian Nahed, Emad Eskandar, and Jean-Valery Coumans declare they have no conflict of interest.





