INTRODUCTION
Intentional insulin overdoses are relatively uncommon, with only 166 cases reported to Canadian poison centres (excluding Quebec) in 2016 (personal communication with Canadian poison centre staff: D Leong [BC], M Mink [AB], M Thompson [ON], A Letarte [QC], and L Mosher [NS]; July 2017). Similarly, U.S. poison centres reported only 592 cases in 2015.Reference Mowry, Spyker and Brooks 1 Although uncommon, severe hypoglycemia necessitating frequent blood glucose measurements, active glycemic control, and, in some cases, admission to an intensive care unit may occur.Reference Lu and Inboriboon 2 , Reference Klein-Schwartz, Stassinos and Isbister 3 Standard therapy includes enteral feeding and intravenous (IV) dextrose infusions; acute episodic hypoglycemia often mandates treatment with concentrated dextrose boluses.Reference Klein-Schwartz, Stassinos and Isbister 3
Octreotide is a semisynthetic, long-acting somatostatin analogue that suppresses endogenous insulin release.Reference Glatstein, Scolnik and Bentur 4 It is commonly used to minimize hypoglycemia following sulfonylurea overdose.Reference Klein-Schwartz, Stassinos and Isbister 3 , Reference Glatstein, Scolnik and Bentur 4 Hypoglycemia from sulfonylurea toxicity is largely the result of endogenous insulin release, triggered by the binding of the drug to ATP-sensitive potassium channels.Reference Glatstein, Scolnik and Bentur 4 , Reference Fuller, Miller and Kaylor 5 Following an insulin overdose, few case reports have proposed the use of octreotide as an adjunct to standard therapy for dextrose-induced episodic hypoglycemia resulting from endogenous insulin release in non-diabetic and non-insulin-dependent diabetic patients.Reference Lu and Inboriboon 2 , Reference Groth and Banzon 6 Purported benefits include reductions in hypoglycemic episodes, IV fluid volume, and total dextrose requirements.Reference Klein-Schwartz, Stassinos and Isbister 3 , Reference Glatstein, Scolnik and Bentur 4 We report a unique case of subcutaneous (SC) octreotide use in a non-diabetic patient following an intentional overdose with insulin aspart.
CASE REPORT
A 41-year-old, non-diabetic woman purchased insulin aspart from an online Canadian pharmacy and injected 300 units SC into a single site on her abdomen with suicidal intent. She also ingested 45 mg of zopiclone, 2 mg of clonazepam, and six vodka coolers. She contacted a friend who subsequently alerted Emergency Medical Services; paramedics identified the product found in the patient’s home as NovoRapid, a brand name of insulin aspart. Approximately 40 minutes after the insulin injection, paramedics found her to be ataxic, lethargic, and diaphoretic, with normal vital signs and an initial glucose of 2.3 mmol/L (41 mg/dL). She immediately received a 50-mL ampoule of 50% dextrose (D50W). During transport to the hospital, a second dose was administered for recurrent hypoglycemia. Upon arrival to the emergency department (ED), her glucose was 8.4 mmol/L (151 mg/dL).
In the ED, 1.5 hours post-injection, her vital signs were blood pressure, 95/51 mm Hg; heart rate, 78/min; respiratory rate, 16/min; oxygen saturation, 94% on room air; temperature, 36.3 C. She was drowsy but responded to questions appropriately. She denied ingesting any other hypoglycemic agents, including sulfonylureas. The rest of her physical examination was unremarkable. Acetaminophen and salicylate concentrations were negative. Laboratory values of note included a serum ethanol concentration of 26 mmol/L (120 mg/dL) and a serum potassium of 2.7 mEq/L, for which she received 40 mmol potassium chloride in 1000 mL of normal saline, infused at 150 mL/h.
During her initial 10 hours in the ED, she received approximately 2000 mL of 10% dextrose solution IV at rates of 250-425 mL/h. An additional 12 ampoules of D50W were administered for recurrent hypoglycemia, arbitrarily defined by the admitting service as glucose <3.8 mmol/L (<68 mg/dL). Eating and drinking were encouraged, but she refused. Total IV fluid administration during the 10-hour period totalled approximately 4000 mL.
At 11.5 hours post-injection, she was assessed by the medical toxicology service and noted to be diffusely edematous. The admitting service was considering placement of a central venous catheter so that lower volumes of more concentrated dextrose solutions could be administered. Upon recommendation by a medical toxicologist, octreotide, 50 mcg SC every 6 hours, was initiated. Her first dose accompanied an additional ampoule of D50W for a blood glucose of 2.9 mmol/L (52 mg/dL) at 12.5 hours post-injection.
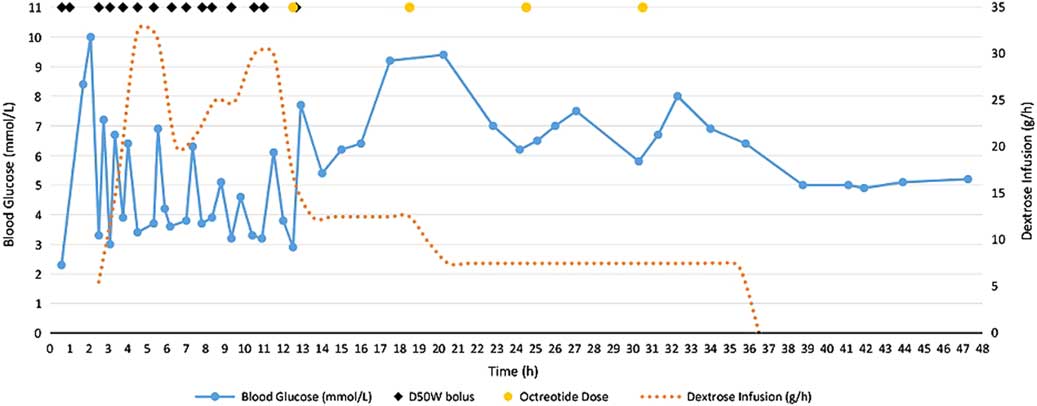
Shortly following the first dose of octreotide, blood glucose control stabilized (Figure 1). No further D50W was required and her dextrose infusion was slowly weaned and discontinued 36.5 hours after her overdose. She received a total of four doses of octreotide and was observed on a medical ward for 48 hours before being transferred to a psychiatric unit.
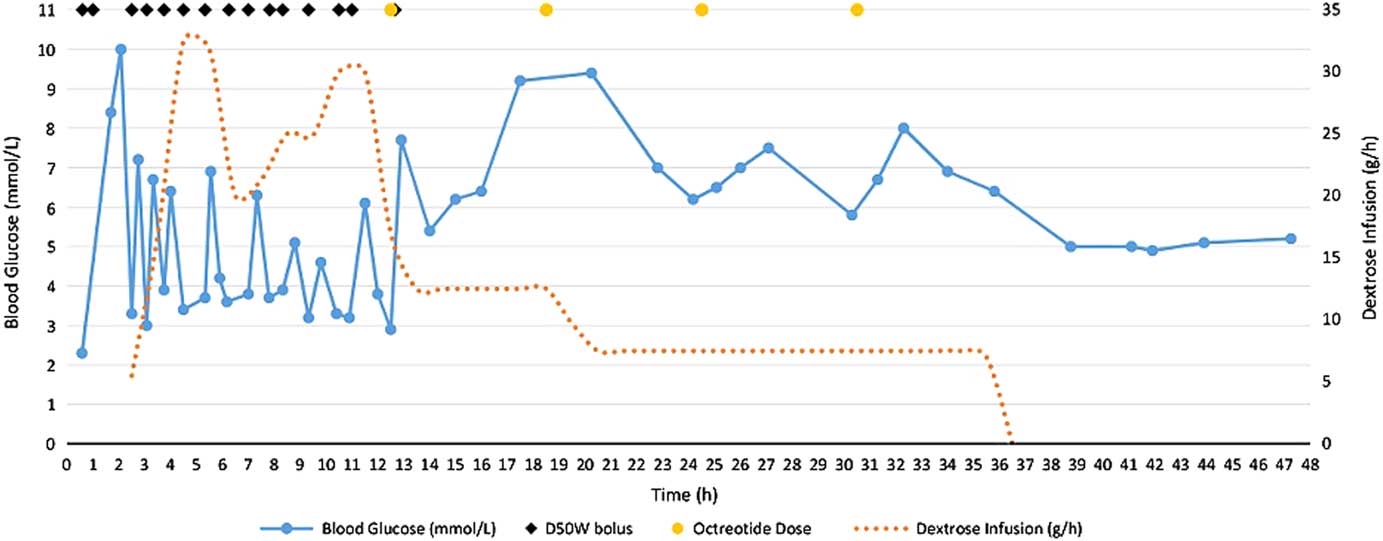
Figure 1 Blood glucose trends and dextrose requirements for 48 hours after intentional insulin aspart overdose.
DISCUSSION
This case report describes a large intentional insulin overdose in a non-diabetic patient causing recurrent hypoglycemia, despite large volumes of IV dextrose. Treatment with SC octreotide was initiated 12.5 hours after an insulin injection, at which point her blood glucose concentrations stabilized, and she tolerated a tapering of her dextrose infusions.
When used therapeutically, insulin aspart has a duration of action ranging from 3 to 5 hours. 7 In overdose, the toxicokinetics of insulin aspart differ vastly from its pharmacokinetics, and hypoglycemia may be prolonged for a number of reasons (Table 1). Consequently, dextrose infusions may be required for multiple days, and higher injected doses appear to correlate with a requirement for longer infusion durations.Reference Lu and Inboriboon 2 , Reference Klein-Schwartz, Stassinos and Isbister 3 , Reference Arem and Zoghbi 11 , Reference Bosse 12 In this case, the large dose and single abdominal injection site were likely the most significant contributors to the prolonged hypoglycemia that occurred.
Table 1 Factors affecting the clinical course of an insulin overdose.
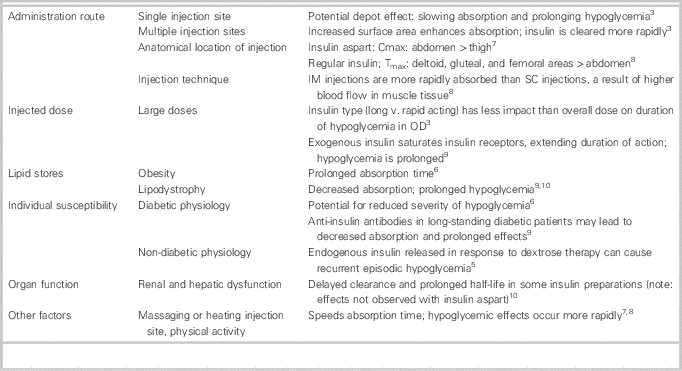
Cmax=maximum concentration; IM=intramuscular; OD=overdose; SC=subcutaneous; Tmax=time to maximum concentration.
The administration of dextrose, either IV or enteral, following an insulin overdose is the mainstay of therapy. Treatment with IV dextrose solutions is not without risks, including phlebitis (risk increases with concentration), hyperglycemia, and complications related to serum hyperosmolality.Reference Klein-Schwartz, Stassinos and Isbister 3 Patients may also develop pulmonary edema, heart failure, and electrolyte abnormalities as a result of fluid overload and osmotic shifts.Reference Klein-Schwartz, Stassinos and Isbister 3 , Reference Groth and Banzon 6 After 10 hours of IV dextrose, our patient developed significant peripheral edema, likely due to a combination of the fluid volume and large sodium and glucose loads administered to an initially euvolemic patient. Considerations were being made to increase the concentration of the dextrose infusion, which would have necessitated the insertion of a central venous catheter for administration.Reference Lu and Inboriboon 2
In overdoses involving insulin secretagogues, such as sulfonylureas and meglitinides, treating hypoglycemia with dextrose boluses can trigger physiologic insulin release in patients with functioning pancreatic cells, complicating the management of these poisonings.Reference Klein-Schwartz, Stassinos and Isbister 3 This same phenomenon, though less well described, can occur in an insulin overdose and likely explains the recurrent hypoglycemia observed in our non-diabetic patient following D50W boluses (see Figure 1).Reference Klein-Schwartz, Stassinos and Isbister 3 , Reference Groth and Banzon 6 Besides oral hypoglycemic agents and insulins, other drug-related causes of hypoglycemia to consider in the differential diagnosis include quinine, sulfonamides, valproic acid, venlafaxine, beta blockers, and salicylates.Reference Bosse 12
Octreotide binds to somatostatin-2 receptors on the beta-cell calcium channel, inhibiting calcium influx. This, in turn, prevents exocytosis of insulin-containing vesicles.Reference Klein-Schwartz, Stassinos and Isbister 3 , Reference Glatstein, Scolnik and Bentur 4 Treatment with octreotide following an insulin overdose may be expected to benefit non-diabetic and non-insulin-dependent diabetic patients whose functional pancreatic beta-cells are capable of releasing endogenous insulin in response to supplemental dextrose.Reference Groth and Banzon 6 Specifically, octreotide may benefit patients susceptible to fluid overload by decreasing the volume of dextrose-containing fluids required to maintain euglycemia.Reference Groth and Banzon 6 No beneficial effect is expected in the insulin-dependent patient with minimal or no endogenous insulin secretion.Reference Fuller, Miller and Kaylor 5 One case report describes the effective use of octreotide in an insulin glargine overdose patient who could not tolerate excess fluid volume due to heart failure and renal impairment.Reference Groth and Banzon 6 The authors reported stabilization of blood glucose concentrations after several doses of octreotide.Reference Groth and Banzon 6
Onset of action of octreotide occurs within 30 minutes of an SC injection, and peak effects are seen at 4-8 hours.Reference Groth and Banzon 6 Our patient achieved euglycemia soon after octreotide initiation, keeping with the predicted clinical effects of the drug. Octreotide is generally well tolerated, with nausea, vomiting, and the injection site pain being the most commonly reported adverse effects.Reference Klein-Schwartz, Stassinos and Isbister 3 , Reference Groth and Banzon 6 , 7 No adverse effects were reported during the treatment of our patient.
A potential downside to octreotide use is that it also suppresses endogenous glucagon secretion, inhibiting the body’s natural biological response to hypoglycemia.Reference Klein-Schwartz, Stassinos and Isbister 3 , Reference Glatstein, Scolnik and Bentur 4 , Reference Fuller, Miller and Kaylor 5 Although it may seem counterproductive to inhibit this action, studies of octreotide use in sulfonylurea toxicity have shown that its administration reduces both endogenous insulin levels and the incidence of recurrent hypoglycemia.Reference Dougherty and Klein-Schwartz 10 In this case, where the patient’s normal physiologic insulin release in response to dextrose administration was presumably complicating her treatment, the authors felt that the benefits of inhibiting insulin secretion outweighed the risks of suppressing glucagon activity.
We recognize that our case report has some limitations. It is possible that, at the time of the octreotide administration, the insulin effects were also starting to wane and that stabilization of blood glucose was due to a dissipating insulin effect. We feel that this is unlikely because Figure 1 clearly shows that the recurrent hypoglycemic episodes occurring after each D50W bolus were not decreasing in frequency or severity prior to the initiation of octreotide. Additionally, the patient had several co-ingestions, including zopiclone, clonazepam, and ethanol. However, zopiclone and clonazepam are not expected to affect blood glucose, and acute ethanol ingestion in a nonalcoholic, such as our patient, would not be expected to cause persistent and recurrent hypoglycemia. Even if mild hypoglycemia did occur as a consequence of alcohol ingestion, recurrent episodes following D50W boluses would not be expected. Finally, the first dose of octreotide was administered at the same time as her final D50W dose, so the full effect of octreotide could not be appreciated until the effects of the dextrose bolus wore off.
Another limitation in our report relates to the lack of supportive laboratory data, specifically C-peptide and proinsulin concentrations. Insulin is secreted from pancreatic beta-cells as proinsulin, which is subsequently cleaved to form insulin and C-peptide. In this case of known exogenous insulin overdose, C-peptide levels could be used as a measurement of endogenous insulin release. Unfortunately, in the region where this patient was treated, C-peptide levels are only processed by an external laboratory once per week, precluding its utilization in the acute care setting. The use of octreotide in future insulin overdose situations should be accompanied by the measurement of C-peptide levels before and after octreotide administration, if available. Although levels would not contribute to the acute treatment of the patient, it could provide an indication of endogenous insulin release. Lastly, because the patient’s history and clinical presentation were consistent with an insulin overdose, we did not send comprehensive drug screens or drug concentrations, specifically oral hypoglycemic assays, to rule out potential co-ingestion. Importantly, a single case report of octreotide use in insulin overdose is insufficient to warrant a change in practice recommendations for the vast majority of patients.
In summary, the majority of insulin overdose presentations will respond to supportive measures with enteral feeding and IV dextrose. In this case, the patient did not tolerate the large fluid volumes required to maintain euglycemia, and glucose concentrations did not stabilize until after octreotide was administered. Further studies and a careful patient selection required before the use of octreotide following insulin overdose in non-diabetic and non-insulin-dependent diabetic patients can be routinely recommended.
CONCLUSION
Emergency physicians should be aware of the severe and refractory hypoglycemia that may occur following an insulin overdose. Although exogenous dextrose administration is the mainstay of therapy, in select non-diabetic patients with normal pancreatic function, endogenous insulin release may lead to recurrent hypoglycemia. In addition, prolonged dextrose infusions are not without risk, including volume overload and peripheral edema. The appropriate use of octreotide in select patients who cannot tolerate or who have complications from exogenous dextrose may help stabilize blood glucose concentrations. Because insulin overdose presentations are relatively uncommon, it is important to involve local poison control centres in the management of these patients.
Competing interests: None declared.