Introduction
The World Health Organization (WHO) defines transient ischemic attack (TIA) as a sudden, focal neurological deficit lasting for less than 24 hours, presumed to be of vascular origin, and confined to an area of the brain or eye perfused by a specific artery. 1 , Reference Albers, Caplan and Easton 2 TIAs are relatively common, having an annual incidence of 68 per 100,000.Reference Hill, Yiannakoulias and Jeerakathil 3 Although relatively benign, given that TIAs cause temporary neurological deficits, they are important because they identify individuals at high risk of subsequent stroke. Previous studies have estimated this risk to be 4%–10% within 7 days of TIA, and increases to 8%–12% by 90 days.Reference Hill, Yiannakoulias and Jeerakathil 3 - Reference Perry, Kerr, Symington and Sutherland 12
Stroke is a serious and significant health and economic burden, because it is the leading cause of adult disability, the second leading cause of dementia, and the third leading cause of death.Reference Johnston 13 - Reference Jorm and Jolley 15 In addition, it results in the use of large amounts of health care system resources.Reference Rosamond, Flegal and Furie 16 Effective prevention targeting those at greatest risk can be expected to provide individual and system benefits.
The ABCD2 Score (age, blood pressure, clinical features, duration of symptoms, and diabetes) developed by Johnston et al. has been used to help identify patients having a TIA who are at high risk of stroke.Reference Johnston, Rothwell and Nguyen-Huynh 17 - Reference Sheehan, Merwick and Kelly 22 Unfortunately, the ACBD2 Score has not performed well in prospective validation (c-statistic: 0.56).Reference Perry, Sharma and Sivilotti 23 We recently conducted a prospective, multicentre study to determine clinical features of TIA patients presenting to emergency departments (EDs), associated with high risk of stroke and to develop a new clinical prediction score for impending stroke.Reference Perry, Sharma and Sivilotti 24 The Canadian TIA Score consists of 13 variables, identifies the risk of subsequent stroke ≤7 days, and quantifies the impending stroke risk following an ED visit for a new TIA.
The objectives of the current study were to assess emergency physician opinions regarding the following: 1) assess their anticipated use of the Canadian TIA Score by emergency physicians; 2) assess the Canadian TIA Score’s component face validity; 3) define the risk strata for stroke within 7 days, and 4) assess the actions required for the Canadian TIA Score dependent upon level of risk for subsequent stroke.
Methods
Study design and participants
This study was a postal survey of emergency physicians in Canada. To be considered for the study, physicians must have been practicing emergency medicine and seeing adult patients. A random sample of 300 emergency physicians was selected from a total of 2,450 registered in the Canadian Medical Directory. A $10 coffee card was given with the first survey to all of the physicians.
Outcome measures
The primary objective for the survey was to determine the optimal cut-points for creating minimal, low, high, or critical risk strata for subsequent stroke within 7 days following TIA diagnosis. We were interested in determining the optimal cut-points that the majority (75%) of emergency physicians would be satisfied with (i.e., the 25th percentile). This percentile was chosen a priori by the study team as a pragmatic value that would include 75% of respondents, whereas the median would satisfy only half of the respondents. This study also investigated physicians’ opinions on the Canadian TIA Score’s component face validity and the management for TIA patients at each risk strata for subsequent stroke within 7 days of TIA diagnosis.
Questionnaire development
We followed the well-known Dillman’s Tailored Design technique for the design of the survey.Reference Sutton, Grimmer-Somers and Jeffries 25 To aid the development of the final survey questionnaire, the survey was developed in three stages: 1) key informant, in-person interviews (pre-survey), 2) cognitive interviews (draft survey), and 3) pilot-testing (final draft survey). The key informant and cognitive interviews were conducted on convenience samples of neurologists and emergency physicians. Key informant interviews (pre-survey) were conducted to establish feasibility of the survey, obtain current knowledge of physicians on TIA, and determine ideal methods on gathering information for the components of the TIA as well as the risk strata. Clarity, comprehensibility, and face validity of the draft survey were evaluated through cognitive interviews. The cognitive interviews were conducted by providing the questionnaire to the physicians and asking them to read aloud their thoughts while completing the survey. In addition, their body language such as facial expressions, pauses, and referrals to previously completed questions were observed and clarified. The pilot survey (final draft survey) was conducted to identify and address any potential problems with our survey implementation procedure or the questionnaire.
The final questionnaire consisted of five sections with a total of 44 questions, and was printed on two single-sided pages (Appendix). The questionnaire consisted of an eligibility question (1 item), demographic and practice setting items (6 items), components of the proposed Canadian TIA Score (13 items), usage of the Canadian TIA Score (1 item), management for TIA patients at each risk strata (19 items), and optimal cut-points for the risk strata (4 items). The cut-points were asked as open-ended percentage questions. The survey materials (final questionnaires, pre-notification letters, and cover letters) were translated by a trained medical translator into French for the French-speaking physicians as identified by the language of correspondence indicator in the Canadian Medical Directory. 26
Survey administration
We mailed the final survey questionnaire to all 300 English and French-speaking emergency physicians in our sample. A pre-notification letter was sent out to all 300 physicians explaining that they had been selected to receive a survey and the importance of their input. A week later, the survey packages consisting of a cover letter, a questionnaire, a prepaid business reply mail envelope, and a coffee card were mailed to all of the physicians. Reminders along with a replacement questionnaire were mailed to the non-respondents every 3 weeks. The final reminder was mailed using Canpar courier service.
The researchers coordinating this study were located at the Ottawa Hospital Research Institute in Ottawa, ON. This study was approved by the Ottawa Health Science Network Research Ethics Board.
Data analysis
Descriptive statistics were used to summarize emergency physician responses. The four possible responses (very important, important, less important, and never important) on the components of the Canadian TIA Score were dichotomized as important (very important or important) or not (less important or never important). The four possible responses (yes, likely, unlikely, no) on whether the physicians will incorporate the proposed Canadian TIA Score in their clinical practice were dichotomized as yes (yes or likely) and no (unlikely or no). The optimal cut-points for the risk strata were presented using frequency distributions and box plots. The Canadian TIA Score’s component face validity and management of TIA patients were presented using bar graphs. Data were analysed using SAS version 9.2 (SAS Institute, Cary, NC, USA).
Results
Respondents
From a total of 300 emergency physicians surveyed, 10 were unreachable because they had moved, and 19 were ineligible because they were no longer practicing or were not seeing adult patients. We received 131 completed surveys from a total of 271 eligible physicians resulting in a response rate of 48.3%.
Demographic information about the respondents is presented in Table 1. A significantly higher proportion (71.8%) of respondents were male. The most common practice location was a teaching hospital (50.4% of respondents) followed by community or district general hospitals with teaching (42.0% of respondents). More than 73.3% of the emergency physicians had been in practice for 10 or more years. A high proportion (96.2%) of the emergency physicians indicated that they would use a validated Canadian TIA Score.
Table 1 Distribution of physician characteristics

SD=standard deviation.
Risk strata cut-points for subsequent stroke within seven days of TIA diagnosis
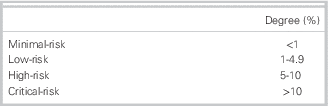
Our results indicate that 75% of emergency physicians classified minimal risk as any cut-point below 1%. Low risk was classified as a score from 1% to 4.9%. High risk was classified as a score from 5% to 10%. Critical risk was defined as any score above 10%. These findings are summarized in Table 2.
Table 2 Degree of risk accepted by 75% of physicians for subsequent stroke ≤7 days
The distribution of emergency physician responses on risk strata cut-points is presented in Figure 1. In general, there is less variability among the emergency physicians on the cut-points up to the low-risk strata. There is, however, a notable variability among the physicians on the cut-points at higher risk strata.

Figure 1 Distribution of Physician Responses on Optimal Cut-Points for Risk Strata.
Component face validity
Figure 2 describes physician attitudes on the importance of select variables for the proposed stroke risk score, including the percentage rated as important for each. The results indicate that more than 75% of physicians thought that the following variables were important in the proposed stroke risk score: atrial fibrillation on electrocardiogram (ECG), history of unilateral weakness, symptoms of first TIA lasted ≥10 minutes, history or exam finding of dysarthria or aphasia (i.e., slurred speech or word finding problems), past medical history of carotid stenosis, infarction (old or new) on CT head, already on any antiplatelet therapy and first ever TIA. Fewer than 75% of physicians thought the following were important: history of gait disturbance, initial diastolic blood pressure at triage ≥110 mm Hg, glucose ≥15 mmol/L, platelets ≥400×109/L, and history of vertigo (as a negative predictor).

Figure 2 Physicians’ views on the importance of select variables in the proposed stroke risk score.
Management given to patients
Our results indicated that the investigations suggested by at least 75% of physicians for each risk stratum were as follows: minimal-risk: obtain an ECG today, obtain brain CT imaging as an outpatient, image the carotid arteries as an outpatient, obtain echocardiogram as an outpatient, and order an outpatient holter cardiac monitoring; low-risk: obtain an ECG today, image the carotid arteries as an outpatient, obtain echocardiogram as an outpatient, and order an outpatient holter cardiac monitoring; high-risk: obtain an ECG today, obtain brain CT imaging today, and put on cardiac monitor for ≥ 2 hours today; critical-risk: obtain an ECG today, obtain brain CT imaging today, image the carotid arteries today, obtain echocardiogram today, and put on cardiac monitor for ≥ 2 hours today. These findings are displayed in Figure 3.

Figure 3 Physician responses on investigation chosen for patients for each stroke risk stratum.
In addition to maintaining a current antiplatelet agent or starting acetylsalicylic acid (ASA), recommended actions by at least 75% of physicians for each risk stratum were as follows: minimal-risk and low-risk: refer patient to rapid outpatient assessment with neurologist; high-risk: start or switch antiplatelet agent to clopidogrel or dipyridamole + ASA, start oral anticoagulation if in atrial fibrillation, start statin class medication, start or optimize control of hypertension, refer patient to neurology today; critical-risk: start or switch antiplatelet agent to clopidogrel or dipyridamole + ASA, start oral anticoagulation if in atrial fibrillation, start statin class medication, start or optimize control of hypertension, admit patient to hospital, and refer patient to neurology today. These findings are displayed in Figure 4. The percent of physicians who would report the patient to the ministry of transportation to suspend licence for each stratum were as follows: minimal-risk: 11.8%; low-risk: 21.2%; high-risk: 73.0; critical-risk: 100.0.

Figure 4 Physician Responses on Management Given to Patients for Each Stroke Risk Stratum.
Discussion
We assessed emergency physician opinions on the proposed Canadian TIA Score to identify patients at high risk of stroke within 7 days of TIA diagnosis. This study shows that a high proportion of emergency physicians is likely to use a validated Canadian TIA Score.
The majority of emergency physicians consider most of the TIA Score’s 13 variables as important. Not all of the variables received the same validity. In particular, elevated blood glucose, elevated platelets, and vertigo were less well-received by the emergency physicians. Although uncontrolled hyperglycemia has been previously demonstrated, it is uncertain whether physicians were less familiar with the risk, or if they were less comfortable with this specific cut-point. Likewise, it is well known that patients with thrombocythemia are at a higher risk of systemic thrombotic disorders, and physicians may have been less aware of this risk, or less comfortable with this specific cut-point. Finally, isolated vertigo is an extremely rare manifestation of TIA or stroke, physicians may not realize how rare isolated vertigo is as a presentation of stroke or TIA, or they may not have realized that patients with other findings besides vertigo will score at a higher risk level than those with isolated vertigo. Educational efforts may be needed if these elements are included in the final validated Canadian TIA Score.
The 2009 American Heart Association recommendations for managing TIA include 1) neuroimaging within 24 hours (preferably MRI) plus noninvasive imaging of the cervical vessels; 2) electrocardiography as soon as possible after TIA, with prolonged cardiac monitoring and echocardiography in patients in whom the etiology is not identified; 3) routine blood tests; and 4) hospitalization for patients presenting within 72 hours of TIA with an ABCD2 Score >2 (two).Reference Easton, Saver and Albers 27
The current cut-point recommendation by the American Heart Association is an ABCD2 Score of 2 or more. Such a cut-point resulted in an excellent sensitivity for stroke at 7 days but poor specificity.Reference Perry, Sharma and Sivilotti 23 According to these guidelines on managing suspected TIA patients, many resources would be used on patients not at risk for stroke, which is not pragmatic or cost-efficient to the health care system. Based on our survey results, physicians are able to define risk strata for impending stroke and have specific management recommendations for each. Therefore, if the Canadian TIA Score is used by emergency physicians, they may be able to more accurately identify those patients at greater risk and allocate health care resources in more effective and efficient ways.
The Canadian Best Practice Recommendations for Stroke Care (2013) suggest a variety of investigations and treatment modalities; however, they are not organized based on specific risk categories. Instead, these recommendations are broad. Physicians treating patients with suspected TIAs are advised to order basic blood work, including electrolytes, glucose, complete blood count (CBC), international normalized ratio (INR), activated partial thromboplastin time (aPTT), creatinine, glomerular filtration rate (GFR), blood urea nitrogen (BUN), lipid profile, liver panel, and troponin. For imaging, all patients are recommended to undergo ECG, chest x-ray, and immediate head CT or MRI if urgently available, as well as vascular imaging of the brain and neck arteries within 24 hours. In terms of acute blood pressure management, there are no definitive recommendations provided for patients presenting with suspected TIAs, because guidelines are listed for only patients who are presenting with suspected stroke. Blood glucose abnormalities should also be checked for and corrected, but, again, this was mentioned specifically for only patients who are presenting with suspected stroke.Reference Lindsay, Gubitz, Bayley and Phillips 28 The Canadian Best Practice Guidelines appear to provide no graded recommendations for patients presented with TIAs. Instead, these are global recommendations, which leave physicians responsible for assessing level of risk for a stroke the patient presents with, in addition to selecting the most appropriate tests based on their own deductions. Given our survey results and proposed tool, physicians are able to define risk strata for impending stroke and have indicated suggested management recommendations for each stratum (i.e., given their knowledge and the existence of current evidence). If adopted into practice, our tool may help Canadian physicians better assess subsequent stroke risk, and this may assist physicians to determine how quickly testing, assessment, and interventions need to be implemented.
The survey was designed following rigorous methodological approaches informed by Dillman’s Tailored Design method.Reference Dillman 29 Our study was conducted on a large, random sample of Canadian emergency physicians. The survey used was optimally developed using key informant and cognitive interviews and subsequently piloted with Ottawa ED physicians. Our study does have potential limitations. It may have been affected by non-response bias due to our 48.3% response rate. Non-responder answers may be different from those responses actually received, which could affect the generalizability of our results. Although there is a chance of a non-response bias, well-done physician surveys often only achieve response rates of around 50%.Reference Perry, Symington and Mansour 30 - Reference Asch, Jedrziewski and Christakis 33 The risk strata cut-offs were determined using the 25th percentiles from the distribution of physician responses to satisfy 75% of physicians. Such cut-offs could be insufficient for up to 25% of physicians. Our respondents were mainly male physicians (71.8%) which was in agreement with what was available on the sample frame (75.3%). 34
Respondents also came from a variety of training backgrounds and clinical centers, where practice and resource availability may differ, and could possibly influence results. Emergency physicians involved with teaching hospitals represented the majority of respondents, at a combined 92%. This population may be different than the majority of practicing ED physicians in Canada, possibly making our results less generalizable. However, our respondents may actually be representative of Canadian ED physicians. The Canadian Medical Directory we selected 300 physicians from is up-to-date, and an appropriate randomization process was followed using computerized randomization. Furthermore, in 1993, a Canadian study reported 65.8% of Canadian ED physicians surveyed worked in teaching hospitals. Because there are more training positions in rural communities for medical students or residents, it is reasonable that this percentage may have increased. Our survey design did not further delineate whether the site was in a large urban setting or within a more rural site, and we did not quantify the number of learners. Finally, there is the possibility of response bias in our respondents. Our rating scales provided equal positive and negative options, but did not include a neutral option, because some more traditional 5- and 7-point Likert scales have. However, our survey design was rigorous in order to discard any potentially leading questions, through the informant and cognitive interviews as well as piloting by Ottawa ED physicians. Finally, at the beginning of the survey, we provided explicit instructions to eliminate any respondents from completing our survey who did not meet our inclusion criteria.
This study demonstrates that Canadian emergency physicians are likely to use a validated Canadian TIA Score. Furthermore, most components of the Canadian TIA Score have high face validity. Risk strata are definable, and physicians are able to agree to clear actions of each risk category.
Competing interests: Authors would like to declare funding from the Canadian Stroke Network Summer Studentship, a grant from the University of Ottawa, Department of Emergency Medicine.
Supplementary Material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/cem.2015.57








