Abstract link: https://www.ncbi.nlm.nih.gov/pubmed/29897851
Full citation: Kuppermann N, Ghetti S, Schunk JE, Stoner MJ, Rewers A, McManemy JK, et al. Clinical trial of fluid infusion rates for pediatric diabetic ketoacidosis. N Engl J Med 2018;378(24):2275–87.
Article type: Therapy
Ratings: Methods – 4/5 Usefulness – 4/5
INTRODUCTION
Background
Prior guidelines for fluid management in pediatric diabetic ketoacidosis (DKA) were based on limited observational data. Rapid fluid administration was thought to cause cerebral injury due to sudden changes in serum osmolality.Reference Hom and Sinert1
Objective
To study the effect of different fluid regimens on neurologic outcomes in pediatric DKA.
METHODS
Design
Multicentre randomized, controlled trial (RCT) (2 x 2 factorial design).
Subjects
Children (0–18 years of age) with DKA.
Subjects were excluded if:
• Glasgow Coma Scale (GCS) ≤ 11
• Pregnancy
• Treated DKA
• Condition impairing mental status
• Specific intravenous therapy required
Intervention
Fast rehydration with 0.45% or 0.9% NaCl (20 mL/kg bolus, replace half of 10% fluid deficit over 12 hours, remaining deficit over following 24 hours).
Comparison
Slow rehydration with 0.45% or 0.9% NaCl (10 mL/kg bolus, replace half of 5% fluid deficit over 48 hours).
Primary outcome
Neurologic decline (GCS < 14 twice consecutively within any hour of first 24 hours of treatment).
Secondary outcomes
Short-term memory loss in first 24 hours; clinically apparent brain injury; follow-up neurologic outcomes.
RESULTS
In total, 1,389 cases from 1,255 patients were included in the study. Ultimately, 1,361 cases were analysed for the primary outcome. No statistically significant differences were observed between groups for either the primary or secondary outcomes. Results are shown in Tables 1 and 2.
Table 1. Mental status change by treatment group
Table 2. Relative risk of mental status change by treatment group
APPRAISAL
Strengths
• Consecutive patient recruitment
• Adequate participant randomization
• Baseline demographic and prognostic factors similar between study groups
• Analysis followed the intention-to-treat principle
• Between group therapy only varied based on treatment group assigned
• Successful follow-up for secondary outcome measures
Limitations
• Low primary outcome event rate predisposing the study to type II error
• Underpowered study due to an over-estimate of the incidence of the primary outcome
• Less than 50% of eligible patients included in the RCT
• 10% of patients were withdrawn by the treating physician
• The study design excluded the sickest DKA patients (GCS ≤ 11)
• Physicians were not blinded to treatment group
• Total of 134 patients enrolled in the study more than once
CONTEXT
Increased rates of cerebral edema were not seen with rapid infusion of hypotonic fluids.Reference Kuppermann, Ghetti, Schunk, Stoner, Rewers and McManemy2 This should challenge the commonly cited “osmotic shift” theory which has long dictated DKA resuscitation guidelines. The fact that some trial results suggest better neurologic outcomes in the rapid infusion group [specifically, the highest rate of GCS decline [4.7%] was seen in the slow 0.9% NaCl arm] warrants re-evaluation of existing hospital treatment protocols, exploration of alternative hypotheses behind DKA-related cerebral edema, and replication of this study design in a sicker cohort of DKA patients.Reference Sperling3
BOTTOM LINE
This was the first well-designed RCT to study the commonly held belief that rapid intravenous infusion can cause cerebral edema in DKA. Although both the lack of physician blinding and the inadequate power to detect between group interaction effects represent significant study limitations, the results of this study have already led to updated clinical practice guidelines.Reference Wolfsdorf, Glaser and Agus4 For pediatric rehydration, it is reasonable to use either 0.45% or 0.9% NaCl and to use a rapid or slow rate of intravenous rehydration (replacing 5%–10% of body weight over 24–48 hours) as detailed in the study protocol.

Abstract link: https://www.ncbi.nlm.nih.gov/pubmed/29897851
Full citation: Kuppermann N, Ghetti S, Schunk JE, Stoner MJ, Rewers A, McManemy JK, et al. Clinical trial of fluid infusion rates for pediatric diabetic ketoacidosis. N Engl J Med 2018;378(24):2275–87.
Article type: Therapy
Ratings: Methods – 4/5 Usefulness – 4/5
INTRODUCTION
Background
Prior guidelines for fluid management in pediatric diabetic ketoacidosis (DKA) were based on limited observational data. Rapid fluid administration was thought to cause cerebral injury due to sudden changes in serum osmolality.Reference Hom and Sinert1
Objective
To study the effect of different fluid regimens on neurologic outcomes in pediatric DKA.
METHODS
Design
Multicentre randomized, controlled trial (RCT) (2 x 2 factorial design).
Subjects
Children (0–18 years of age) with DKA.
Subjects were excluded if:
• Glasgow Coma Scale (GCS) ≤ 11
• Pregnancy
• Treated DKA
• Condition impairing mental status
• Specific intravenous therapy required
Intervention
Fast rehydration with 0.45% or 0.9% NaCl (20 mL/kg bolus, replace half of 10% fluid deficit over 12 hours, remaining deficit over following 24 hours).
Comparison
Slow rehydration with 0.45% or 0.9% NaCl (10 mL/kg bolus, replace half of 5% fluid deficit over 48 hours).
Primary outcome
Neurologic decline (GCS < 14 twice consecutively within any hour of first 24 hours of treatment).
Secondary outcomes
Short-term memory loss in first 24 hours; clinically apparent brain injury; follow-up neurologic outcomes.
RESULTS
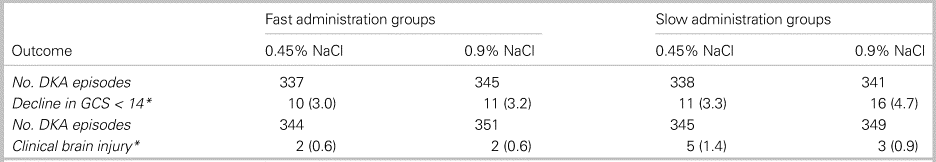
In total, 1,389 cases from 1,255 patients were included in the study. Ultimately, 1,361 cases were analysed for the primary outcome. No statistically significant differences were observed between groups for either the primary or secondary outcomes. Results are shown in Tables 1 and 2.
Table 1. Mental status change by treatment group
*Reported as number of events and as a percentage; no statistically significant differences observed.
Table 2. Relative risk of mental status change by treatment group
CI = confidence interval; NSS = not statistically significant; RR = relative risk.
APPRAISAL
Strengths
• Consecutive patient recruitment
• Adequate participant randomization
• Baseline demographic and prognostic factors similar between study groups
• Analysis followed the intention-to-treat principle
• Between group therapy only varied based on treatment group assigned
• Successful follow-up for secondary outcome measures
Limitations
• Low primary outcome event rate predisposing the study to type II error
• Underpowered study due to an over-estimate of the incidence of the primary outcome
• Less than 50% of eligible patients included in the RCT
• 10% of patients were withdrawn by the treating physician
• The study design excluded the sickest DKA patients (GCS ≤ 11)
• Physicians were not blinded to treatment group
• Total of 134 patients enrolled in the study more than once
CONTEXT
Increased rates of cerebral edema were not seen with rapid infusion of hypotonic fluids.Reference Kuppermann, Ghetti, Schunk, Stoner, Rewers and McManemy2 This should challenge the commonly cited “osmotic shift” theory which has long dictated DKA resuscitation guidelines. The fact that some trial results suggest better neurologic outcomes in the rapid infusion group [specifically, the highest rate of GCS decline [4.7%] was seen in the slow 0.9% NaCl arm] warrants re-evaluation of existing hospital treatment protocols, exploration of alternative hypotheses behind DKA-related cerebral edema, and replication of this study design in a sicker cohort of DKA patients.Reference Sperling3
BOTTOM LINE
This was the first well-designed RCT to study the commonly held belief that rapid intravenous infusion can cause cerebral edema in DKA. Although both the lack of physician blinding and the inadequate power to detect between group interaction effects represent significant study limitations, the results of this study have already led to updated clinical practice guidelines.Reference Wolfsdorf, Glaser and Agus4 For pediatric rehydration, it is reasonable to use either 0.45% or 0.9% NaCl and to use a rapid or slow rate of intravenous rehydration (replacing 5%–10% of body weight over 24–48 hours) as detailed in the study protocol.
Competing interests
None declared.