CLINICIAN'S CAPSULE
What is known about the topic?
Benzodiazepine-resistant alcohol withdrawal syndrome is not well defined in the literature.
What did this study ask?
We assessed clinicians’ personal definition of resistant alcohol withdrawal with a survey-based approach.
What did this study find?
High benzodiazepine dosage (40 mg/hr of diazepam equivalents) was considered a determinant for defining resistant alcohol withdrawal.
Why does this study matter to clinicians?
High benzodiazepine usage should prompt clinicians to suspect resistant alcohol withdrawal syndrome and add another molecule such as phenobarbital.
INTRODUCTION
Alcohol withdrawal is a common problem in emergency departments.Reference Long, Long and Koyfman1,Reference Rehm, Mathers and Popova2 Although benzodiazepines are the first-line treatment,Reference Mayo-Smith3,Reference Lejoyeux, Solomon and Ades4 some withdrawal patients are poorly responsive to benzodiazepines. This entity is often named benzodiazepine-resistant or resistant alcohol withdrawal. Unfortunately, there is no standard definition of resistant alcohol withdrawal. Hack et al. suggested a combination of abnormal vital signs and a cumulative dose of more than 200 mg of diazepam over 3 hours.Reference Hack, Hoffmann and Nelson5 However, these criteria were never validated or subjected to expert consensus. Also, the definition of abnormal vital signs was imprecise. All subsequent definitions of resistant alcohol withdrawal seem to be variations on Hack's initial efforts. Among these, one suggests that more than 10 mg of lorazepam in 1 hour or more than 40 mg in 4 hours would define benzodiazepine resistance.Reference Hughes6 A recent retrospective study defined benzodiazepine resistance based on three different criteria: 200 mg of diazepam in 4 hours; greater than 40 mg of diazepam in 1 hour; or an individual dose of greater than 40 mg of diazepam required to control agitation.Reference Benedict, Wong and Cassidy7 A formalized definition of resistant withdrawal might standardize future research by improving comparability of patients, which ultimately could improve the assessment of interventions on relevant clinical outcomes. The present study assesses clinicians’ personal definitions of resistant alcohol withdrawal with a survey-based methodology.
METHODS
Study design and population
We designed a 25-item open cross-sectional survey assessing two different aspects of alcohol withdrawal among emergency physicians and toxicologists. The first part assesses clinicians’ personal definition of resistant alcohol withdrawal. The questionnaire was imported on the SimpleSurvey platform, a web-based online survey tool. It was displayed on three pages; the first was the consent form, and the others were 13- and 12-item questionnaires, respectively. The survey link was available from July 5, 2017, to September 18, 2017. A convenience sample of associations was based on their willingness and costs to deploy the survey.
The administrators of the Canadian Association of Emergency Physicians (n = 1,447), the European Association of Poison Centres and Clinical Toxicologists (n = 291), the American Academy of Clinical Toxicologists (n = 504), the Asian Pacific Association of Medical Toxicologist (n = 344), the American College of Medical Toxicology (n = 680), and the Association des Pharmaciens d’Établissements de Santé of Quebec (n = 1,978) sent a survey link to their membership. A single reminder was sent 2 weeks before closing the survey. Participation was voluntary. Due to known cross-memberships, eligible respondents were asked to complete the survey once only. Only physicians, pharmacists, or other clinicians managing alcohol withdrawal were eligible to participate. Informed consent was obtained at the beginning of the survey. Answers were anonymous. Ten minutes was the estimated time to complete the survey. This study was approved by our institutional Research and Ethics Board. The CHERRIES checklist was used to report the results.Reference Eysenbach8
Survey design
The questionnaire's content was based on a literature review performed by local experts with backgrounds in clinical research and toxicology. The questionnaire was developed in English and French and was piloted among five local emergency physicians. Written feedback was requested and received by email. Respondents were asked to rank from 1 to 3, three literature-based definitions of resistant alcohol withdrawal, with choice 1 being their preference. Respondents were required to comment on whether they considered these definitions equivalent. Additionally, they could specify their own criteria for resistant alcohol withdrawal. Answers could be reviewed and changed using the “Back” button at any time before submitting their questionnaire. The survey questions are in supplementary material Appendix 1.
Data analysis
All answers were collected with the SimpleSurvey platform and extracted on an Excel spreadsheet (Microsoft Corp, Redmond, WA). An independent statistician eliminated duplicates by comparing IP addresses and demographics; only identical matches were deleted. We summarized categorical responses with counts and proportions. Median and interquartile ranges (IQRs) were calculated for the continuous responses. Benzodiazepine equivalence was defined as diazepam 5 mg = lorazepam 1 mg to express diazepam dosing equivalents (DEQ).Reference Dundee, McGowan, Lilburn, McKay and Hegarty9 Statistical analyses were performed with R studio version 1.1.447 (RStudio, Inc., Boston, MA). The response rate could not be accurately estimated because an unknown proportion of recipients received more than one invitation due to overlap in membership/mailing lists. We therefore defined the participation rate as the proportion of respondents who agreed to participate divided by the number of times the survey link was opened. Surveys that were not completed for at least one question were excluded.
RESULTS
Respondent characteristics
The response rate was 8.6% (453/5244), the participation rate was 48.5% (453/934), and the completion rate was 70.4% (319/453). Respondents totalling 384 provided adequate data for analysis; 302 (78.6%) were attending physicians, 344 (89.6%) had a full-time practice, and 270 (70.3%) were practising emergency medicine. Most respondents (73.7%) were practising for less than 20 years, and 223 respondents (58.1%) treated alcohol withdrawal patients at least once per week. Full respondent characteristics are shown in the supplementary material, Appendix Table 1.
Resistant alcohol withdrawal definition
Table 1 shows the literature-based definitions. Our survey used Benedict's definition as included in the research protocol available on www.clinicaltrial.gov, which was different in the final publications.10 We referred to it as the protocol number definition in the survey. Resistant alcohol withdrawal was described as a high benzodiazepine dosage (i.e., diazepam 40 mg in 2 hours) by 244 respondents (63.5%) followed by seizures by 98 respondents (25.5%). These criteria were not mutually exclusive. Among the 146 respondents who quantified “high-dose benzodiazepine,” the median dose was 40 mg per hour (IQR; 20–50) of DEQ. See Table 2 for the full distribution of criteria surveyed.
Table 1. Definitions of resistant alcohol withdrawal

Table 2. Distribution of criteria defining resistant alcohol withdrawal
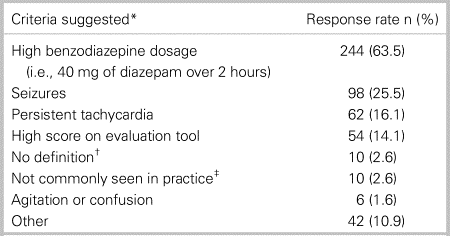
*Criteria not mutually exclusive; †Respondents have no definition of resistant alcohol withdrawal; and ‡Respondents do not see resistant alcohol withdrawal often in their practice.
The number of clinicians who ranked the three literature-based, resistant alcohol withdrawal definitions were Hack, 103 (26.8%); Benedict, 108 (28.1%); Hughes, 109 (28.4%); and 65 (16.9%) did not respond; 222 (57.8%) respondents said these definitions were not equivalent.
DISCUSSION
Clinicians’ individual definitions of resistant alcohol withdrawal are largely based on the total benzodiazepine dose required to control symptoms. The median hourly dose provided by respondents is consistent with the literature-based definitions. However, no single definition of resistant alcohol withdrawal was preferred, because answers were distributed equally over notably distinct definitions.
This survey raises questions whether variability among clinicians in diagnosing resistant alcohol withdrawal affects patient outcome. The IQR calculated for high benzodiazepine dose partially illustrates that variation. Our results begin a discussion to improve understanding this entity. Resistant alcohol withdrawal should be suspected when using more than 40 mg of DEQ per hour and when the patient is worsening or not improving, despite closely repeated doses (seizures, persistent tachycardia, high score on assessment tool, and so on). This relatively new entity needs to be formally characterized in cohort studies to improve our understanding. Expert consensus could provide some definitional guidance to help future studies assess therapeutic interventions.
Strengths and limitations
Although we were unable to calculate cross-membership and ineligibility of individuals who received the link, we screened a large sample of clinicians using a rigourous method and provided a conservative participation rate. We pretested the questionnaire and allowed respondents to add information to their answers if desired. The dropout rate was 15.2%. The same link included a second survey addressing different clinical questions about alcohol withdrawal, which will be discussed separately, and might have prolonged the survey duration contributing to dropout. Deployment during summer months in the Northern hemisphere could have limited the response rate. Multiple reminders could have improved the response rate.
Additionally, we surveyed members of clinical toxicology and emergency medicine associations, which may limit the generalization of our results. However, alcohol withdrawal is often diagnosed and treated in emergency departments with the assistance of clinical toxicologists.Reference Hoskins and Benger11,Reference Cherpitel and Ye12 A selection bias may have been created by using a convenience sample of associations. Also, the term persistent tachycardia was not clearly defined, which may have led to wide interpretation. We were unable to find previous works that either discussed heart rate range for persistent tachycardia or other addressed clinician opinions on this topic.
CONCLUSION
Resistant alcohol withdrawal is an ill-defined clinical entity of unknown prevalence, defined by high-dose benzodiazepines (more than 40 mg of DEQ per hour), seizures, and tachycardia. We hope our findings guide future consensus work towards a formalized definition that allows future standardized research to ultimately improve patient care in terms of a reduction in mortality, morbidity, and health system costs.
SUPPLEMENTARY MATERIAL
The supplementary material for this article can be found at https://doi.org/10.1017/cem.2019.421.
Competing interests
None declared.




