Introduction
Background
Chest pain and shortness of breath are two of the most common presenting patient complaints in the emergency department (ED).Reference Brown, Braithwait and Perina 1 Pulmonary embolism is a potentially lethal yet treatable condition that must be considered in patients with these complaints.Reference Dismuke and Wagner 2 – Reference Silverstein, Heit and Mohr 4 It has an annual incidence of 21 to 69/100,000 population, is the third leading cause of cardiovascular mortality in North America, and causes 5%−10% of in-hospital deaths.Reference Dismuke and Wagner 2 The per-person lifetime incidence of pulmonary embolism is approximately 5%.Reference Dismuke and Wagner 2 – Reference Nordstrom and Lindblad 5
The diagnosis of pulmonary embolism is important, given that undiagnosed pulmonary embolism is believed to have a mortality rate approaching 30%. This falls to less than 8% if pulmonary embolism is diagnosed and appropriately treated.Reference White 6 – Reference Alpert, Smith and Carlson 9 Diagnosing pulmonary embolism can be challenging because it is in the differential diagnosis of many common clinical presentations, including chest pain, shortness of breath, and hemoptysis. Studies suggest that only 8%−30% of patients suspected of having a pulmonary embolism actually have the condition.Reference Kruip MJHA and van der Heul 10 – Reference Hull, Hirsh and Carter 13 As a result, many patients without pulmonary embolism are needlessly anticoagulated, often due, in part, to delays in diagnostic imaging and obtaining a definitive diagnosis. Such patients may undergo extensive testing or hospitalization.
Currently, a myriad of clinical decision tools with variability in their interpretation exists for pulmonary embolism.Reference Wells, Ginsberg and Anderson 12 , Reference Wicki, Perneger and Junod 14 , Reference Le Gal, Righini and Roy 15 Existing rules include various objective measures, but none include a quick, easy, objective bedside measurement. The performance of a standardized 3-minute walk test to obtain an ambulatory oxygen saturation and heart rate has been previously studied in other disease processes.Reference Pan and Stiell 16 The 3-minute walk test has been found to be a simple, low-cost test, which is easy to conduct in a busy clinical setting for the diagnostic management of chronic obstructive pulmonary disease and heart failure.Reference Pan and Stiell 16 , Reference Stiell, Clement and Brison 17 The objective of this study was to assess the utility of a change in ambulatory oxygen saturation and ambulatory heart rate measurement by means of a standardized 3-minute walk test, for the diagnosis of pulmonary embolism.
Materials and Methods
Study design
We conducted a prospective cohort study of patients with a suspected (based on physician gestalt) or recently confirmed (based on imaging studies) pulmonary embolism. All participants underwent a standardized 3-minute walk test. Strict stopping rules were used, and participants had the option to stop the test at any point for any reason beyond those listed in the stopping rules. The results of the walk test were not provided to clinicians unless a diagnostic and/or therapeutic decision was already made. The results of the 3-minute walk test were not incorporated into the ED care and management of participants. The research ethics board of the Ottawa Hospital approved the study. Verbally informed consent was obtained from all participants.
Study setting
The study was conducted in the EDs of two sites of the Ottawa Hospital—an adult, tertiary-care institution affiliated with the University of Ottawa. The involved EDs have an annual census of approximately 140,000 visits. Patients were also recruited at the Ottawa Hospital Thrombosis Clinic. This clinic is responsible for the diagnosis and management of all suspected or confirmed pulmonary embolisms in the greater Ottawa area. Several regional EDs, including those of the Ottawa Hospital, send all confirmed and many suspected patients with pulmonary embolism to this clinic for diagnosis (when unestablished) and further management of pulmonary embolism. The participants enrolled at the thrombosis clinic were patients assessed in an ED during the previous day. Most patients assessed in the clinic did not yet have definitive imaging to establish the diagnosis of pulmonary embolus.
Selection of participants
Adult patients over the age of 18 years who were physically able to participate in a walk test and who received, or were designated to receive, diagnostic imaging to assess for pulmonary embolism were eligible. We excluded patients who had a diagnosed pulmonary embolism in the past 3 months, were nonambulatory (e.g., paralysed, or in a wheelchair), required supplemental oxygenation during their ED visit, were receiving treatment for confirmed venous thromboembolism (anticoagulants or inferior vena cava filter), required therapeutic anticoagulation for other indications at the time of enrolment, or had any contraindications to the computed tomography pulmonary angiogram (e.g., renal impairment, contrast allergy).
The decision to initiate treatment was made by attending emergency physicians, based on positive imaging results completed in the ED and/or by risk stratification (by specific risk score or physician judgement), which in the case of high-risk patients typically involved commending treatment for pulmonary embolism and then referred to the thrombosis clinic. The decision to continue or discontinue treatment was made independently at the discretion of the attending physician or supervised resident at the Ottawa Hospital Thrombosis Clinic.
Methods and measurements
Trained research assistants were deployed in both the ED and thrombosis clinic to screen for potential participants. We opted to use this clinic as an enrolment site in order to increase our enrolment rate. A paid research assistant covered the thrombosis clinic from Monday to Friday. In the ED, study enrolment occurred 7 days a week from 8:00 am to 8:00 pm between May and November 2012. A small number of days were not covered due to lack of staffing. ED coverage was accomplished using a medical student volunteer and the principal investigator. Treating physicians were able to recommend patients for study enrolment. The 3-minute walk-test protocol was as follows: initial baseline vitals were taken (i.e., heart rate, oxygen saturation level, and respiration rate), and patients were asked to walk for a fixed period of 3minutes regardless of the distance covered on ground level within the ED or corridor of the thrombosis clinic.Reference Pan and Stiell 16 They were permitted to use any walking aids, if applicable; otherwise, patients walked without any assistance and were on room air. Patients were accompanied by a research assistant who was responsible for recording oxygen saturation, heart rate, and respiration rate on a case record form at baseline. Continuous recordings were taken during the walk test with electronic printouts from the equipment used. Peak changes during the walk test as well as at 1-minute post-walk test were recorded. The participant’s respiration rate was assessed only pre- and post-walk test because it was difficult to have an accurate peak respiratory rate during ambulation with the study monitor that was used. We allowed the participants to recover for a period of 1minute before checking the post-walk test vital signs. The research assistant carried all necessary equipment, including the pulse oximeter (model 504-DXP; Criticare Systems Inc., Waukesha, WI, USA), leaving the patients free to ambulate independently at their own pace.
The walk test was discontinued if patients experienced worsening dyspnea, had an oxygen saturation less than 86% for 30 seconds, had a heart rate higher than 110 for heart failure patients or 120 for chronic obstructive pulmonary disease patients for greater than 60 seconds, complained of new or worsening chest pain, or if they requested the discontinuation for any reason, as previously published.Reference Pan and Stiell 16 Discontinued walk tests were considered a positive test if any of the stopping rules were implemented, but not in the case of patient requests. Standardized data collection forms were used. The study authors collected outcome data and demographics using an additional study form. Data were entered into a computerized database using SPSS version 20.1 software (IBM Corporation, Chicago, IL, USA).
Outcome measures
The primary outcome of interest was pulmonary embolism. This was defined as any pulmonary embolism treated by thrombosis physicians following confirmatory diagnostic testing. Diagnostic confirmation was made using computed tomography pulmonary angiogram and included any segmental, subsegmental, or saddle embolism. Ventilation perfusion scans followed the currently accepted standard criteria,Reference Perrier, Desmarais and Miron 18 and intermediate or high probability results were deemed to be a positive outcome. Compression ultrasound to diagnose deep vein thrombosis (DVT) utilized The Ottawa Hospital’s protocol, which looks for non-compressibility at the trifurcation of the popliteal vein or above or any new non-compressibility at the trifurcation of the popliteal vein or above if prior imaging was made available. A positive DVT study in a patient suspected of pulmonary embolism was also deemed to be a positive outcome.
The primary outcome, pulmonary embolism, was assessed in all enrolled patients by the study authors, based on predefined diagnostic imaging criteria. Final radiology reports were used to confirm the presence of a pulmonary embolism. Indeterminate results were classified as no pulmonary embolism unless the thrombosis physician treated the patient as having a pulmonary embolism.
Sample size calculation
Diagnostic tests should have a high sensitivity to ensure that patients with pulmonary embolism receive treatment, along with specificity as high as possible to maintain the utility of the test.Reference Miniati, Monti and Bottai 19 We decided a priori that we sought to determine whether the sensitivity of an ambulatory oxygen saturation and ambulatory heart rate test exceeded 70%. However, conservatively assuming that 30% of suspected pulmonary embolisms are confirmed and that the sensitivity of the walk test results exceeds 80%, we sought to show that the lower bound of the confidence interval (CI) would be >70%±10%. Based on this, we determined that a sample size of 270 was necessary to obtain the lower level CI.
Data analysis
Data were placed into standard two-by-two tables to calculate exact binomial 95% CIs for sensitivity, specificity, and likelihood ratios of the walk test for identifying patients with pulmonary embolism. Descriptive and univariate analyses with students’ two-sided t-test and χ-square tests were conducted to determine cut-points with a sensitivity of ≥70%, following the optimal cut-point that was then determined. Potential cut-points were determined based on clinically meaningful and easily remembered whole numbers.
Results
Characteristics of study subjects
Of the 123 patients assessed for eligibility, 114 were recruited (Figure 1). Of these, 30 (26.3%) were ultimately diagnosed with pulmonary embolism. There were 75 (65.8%) participants who had a positive walk test. Only one participant (0.9%) was treated for a pulmonary embolism, despite having indeterminate imaging results. This individual was known to have a recurrent thromboembolic disease with lupus anticoagulant along with a positive walk test. Figure 1 provides details for the nine patients who were assessed for eligibility but were not enrolled.

Figure 1 Study Enrollment.
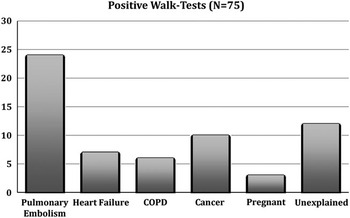
Figure 2 Breakdown of Positive Walk-tests.
Baseline characteristics of the study groups, stratified by outcome, are presented in Table 1. Of the alternative diagnoses for a positive walk test, heart failure and chronic obstructive pulmonary were most common.
Table 1 Baseline characteristics of patient groups, stratified by outcome.

* The Ottawa Hospital General Campus, The Ottawa Hospital Civic Campus, The Ottawa Hospital Thrombosis Clinic all signify different clinical areas of recruitment.
† COPD – chronic obstructive pulmonary disease
‡ DVT/PE – deep vein thrombosis/pulmonary embolism, previous patient history
§ Ultrasound (US) – Compression US revealing: i) non-compressibility at the trifurcation of the popliteal vein or above or ii) new non-compressibility at the trifurcation of the popliteal vein or above if prior imaging is available
¶ Stopping rules – oxygen saturation less than 86% for 30 seconds, a heart rate higher than 110 for heart failure patients or 120 for COPD patients or greater than 60 seconds, complaints of chest pain, or at the patient’s request for any reason
We calculated mean values of the ambulatory walk tests, and these are stratified by outcomes and presented in Table 2. The mean value of an ambulatory heart rate among all participants was 100.6 beats per minute (BPM), and the mean ambulatory oxygen saturation level among all participants was 93.6%.
Table 2 Mean values for walk-test vital signs stratified by outcome.

Main results
An ambulatory oxygen saturation absolute drop of ≥2% and an ambulatory heart rate change of >10 BPM were the optimal cut-points. The majority of the positive walk tests in the study populations were attributed to pulmonary embolism, but there were also positive walk tests in patients who did not have a pulmonary embolism (Figure 2). Of these two assessments, the ambulatory heart rate increase of >10 BPM was superior, with a sensitivity of 96.6% (95% CI 83.3 to 99.4) and specificity of 31.0% (95% CI 22.1 to 45.0) for pulmonary embolism (Table 3). An ambulatory oxygen saturation absolute drop of ≥2% had a sensitivity of 80.2% (95% CI 62.7 to 90.5) and a specificity of 39.3% (95% CI 29.5 to 50.0) for pulmonary embolism.
Table 3 Test characteristics (ambulatory).
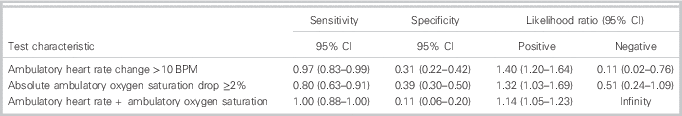
Combining an ambulatory heart rate change of >10 BPM with an absolute change in ambulatory oxygen saturation ≥2% yielded a sensitivity of 100.0% (95% CI 88.7 to 100.0) with a specificity of 11.9% (95% CI 6.6 to 21.0) for pulmonary embolism.
Limitations
This study was conducted at two ED sites, and we had relatively few patients with pulmonary embolism in our study, which potentially limits the generalizability of the results. The inclusion criteria used physician gestalt for many patients in whom pulmonary embolism was not yet confirmed. An ideal assessment would require the application of the 3-minute walk test to all comers in the ED with clearly defined inclusion criteria. Our recruitment model could have led to an observation bias. The research assistants conducting the walk test may have looked more cautiously at the patients during the walk test. This has the potential to lead to more patients with a known diagnosis (of pulmonary embolism) as having a positive walk test. However, we used predefined criteria for what a positive walk test would constitute and included a strategy that involved continuous monitoring during the walk test. This allowed us to review the study monitor electronic printouts afterward to reconfirm the peak vital signs, so we believe that the potential bias we describe had minimal impact on the study and our assessments. Additionally, the incidence of pulmonary embolism in our subset was 26.3%, which was very similar to the reported incidence of 30.0%.Reference White 6 – Reference Carson, Kelley and Duff 8
Several patients had other pre-existing diagnoses, in particular, heart failure and chronic obstructive pulmonary disease. Both illnesses are known to have the potential to result in a positive walk test.Reference Pan and Stiell 16 This may have led to an over-representation in the number of positive walk tests due to etiologies other than pulmonary embolism. Fortunately, our study population had a relatively low proportion with coexisting medical illnesses. A total of 13 (11.4%) participants had a diagnosis of heart failure and/or chronic obstructive pulmonary disease. Thus, we believe that any impact that this has had on our results was unlikely to be substantial. Moreover, both heart failure and chronic obstructive pulmonary disease have historical, physical, and diagnostic testing unique to them and are less likely to obscure the physician’s ability to include or exclude them as a diagnosis when encountered, likely accounting for a very low number of these patients in our study.
During our sample size calculation, we determined that 270 patients were required. However, our study was stopped after 123 patients were enrolled because of financial restrictions. Despite this low sample size, we were still able to derive sensitive and clinically meaningful results.
Finally, the use of noninvasive diagnostic tests as part of the reference standard could be considered a limitation in this study. Although pulmonary angiography remains the gold standard for the diagnosis of pulmonary embolus, no participant in our study underwent one. The use of noninvasive diagnostic tests for the diagnosis or exclusion of pulmonary embolism has become the clinical standard since the PIOPED II trial.Reference Stein, Fowler and Goodman 20
Discussion
In this prospective diagnostic cohort study, we found that change in ambulatory heart rate and change in ambulatory oxygen saturation are both sensitive measurements in the workup of pulmonary embolism. Of the two assessments, an ambulatory heart rate difference >10 BPM from baseline was the superior measurement. However, combining a drop in ambulatory oxygen saturation with a heart rate change >10 BPM yielded the highest sensitivity.
A number of clinical decision rules have been derived to assess the pretest probability in patients suspected to have pulmonary embolism. 11 , Reference Le Gal, Righini and Roy 15 , Reference Perrier, Desmarais and Miron 18 , Reference Rodger, Jones and Rasuli 21 – Reference Rodger, Makropoulos and Turek 35 The three most widely used clinical decision rules are the simplified Wells score, the revised Geneva score, and the pulmonary embolism rule-out criteria (PERC) rule. The simplified Wells score comprises seven variables and has been derived and validated in the North American population.Reference Wells, Anderson and Rodger 28 , Reference Arena and Sietsema 38 The revised Geneva score, which consists of a series of similar measures to the simplified Wells score, contains nine variables encompassing risk factors, symptoms, and clinical signs and has also been found to have a high degree of reliability.Reference Wells, Anderson and Rodger 28 The PERC rule, which was designed to exclude pulmonary embolism without further testing, consists of eight variables, all of which need to be met in order to conclude that a <2% venous thromboembolic rate exists. The simplified Wells score includes a subjective criterion, which may be loosely interpreted: “pulmonary embolism is the most likely diagnosis.” This has resulted in criticism that there can be variation from clinician to clinician in score calculation.Reference Wells, Anderson and Rodger 26 – Reference Stein and Henry 29 Consequently, these rules are often underutilized by practicing clinicians.Reference Stein and Henry 29 – Reference Clark, Votteri and Ariagno 30 This may lead to inappropriate management with resultant increased morbidity and mortality.Reference Roy, Meyer and Vielle 32
All existing decision-making rules include stationary vital sign measurements.Reference Hull, Hirsh and Carter 13 , Reference Perrier, Desmarais and Miron 18 , Reference Stein, Goldhaber and Henry 34 – Reference Rodger, Makropoulos and Turek 35 To the best of our knowledge, ours is the first study to consider the diagnostic power of ambulatory vital signs in an ED setting. The use of an oxygen saturation level in pulmonary embolism has been looked at in a recent study, showing that a pulse oximetry cut-off of 95% room air oxygen saturation at sea level can effectively differentiate patients with pulmonary embolism into high- and low-risk groups.Reference Perrier, Roy and Sanchez 33 Another study evaluated the benefit of a treadmill exercise oxygen saturation test, by way of respiratory gas exchange analysis to help diagnose pulmonary embolism in locations where immediate imaging is not available.Reference Topilsky, Courtney and Hayes 37 The authors concluded that exercise-induced desaturation might be sensitive and specific for diagnosing pulmonary embolism. Although the results of this study were compelling, its results are not feasible for application in a clinical setting because advanced equipment and laboratory analysis were used. Although previously published papers support the physiology of ambulatory oxygen saturation and heart rate,Reference Topilsky, Courtney and Hayes 37 , Reference Arena and Sietsema 38 none have considered this in the workup of pulmonary embolism in an ED setting. Our study has identified two new objective measurements that may help improve the sensitivity of pre-existing decision rules.
Future research must look at the implications of using ambulatory vital signs (heart rate difference and oxygen saturation drop) together with pre-existing rules to determine whether overall diagnosis and subsequent morbidity and mortality are affected.
In summary, our study found that an ambulatory heart rate change >10 BPM or a ≥2% absolute decrease in ambulatory oxygen saturation from baseline during a standardized 3-minute walk test is highly correlated with pulmonary embolism. Although the findings appear promising, neither of these variables can currently be recommended as a screening tool for pulmonary embolism until larger prospective studies examine their performance either alone or with pre-existing rules.Reference Stein and Henry 39 , Reference Freitas and Sarosi 40
Competing interests
None declared.
Grant funding: Provided by the Department of Emergency Medicine and Department of Medicine, The Ottawa Hospital, University of Ottawa, ON.
Acknowledgements: QA and JP conceived the study, designed the trial, and obtained research funding. JP, MR, and IS supervised the conduct of the trial and data collection. QA and SM undertook recruitment of participating centres and patients and managed the data, including quality control. JP, MR, and IS provided statistical advice on study design. JP and QA analysed the data. QA drafted the manuscript, and all authors contributed substantially to its revision. QA takes responsibility for the paper as a whole.
Presentation: Presented at the Canadian Association of Emergency Physicians (CAEP) 2013 Annual Scientific Meeting, Vancouver, BC, Canada.







