Introduction
Leaf beetles are drivers of ecological change in terrestrial biomes, acting as plant pests, biological control agents, and elsewhere as abundant herbivores (Myers and Sarfraz Reference Myers and Sarfraz2017). Québec, Canada has a tradition of collaboration between professionals and skilled amateur collectors in documenting its insect fauna, including economically important species (e.g., de Tonnancour et al. Reference de Tonnancour, Anderson, Bouchard, Chantal, Dumont and Vigneault2017). Here, we report establishment of two additional species of adventive Chrysomelidae found by independent scientific collectors in and near cities in Québec.
The first, Cryptocephalus moraei (Linnaeus, 1758) (Coleoptera: Cryptocephalinae), is native throughout Europe and western Asia (Lopatin et al. Reference Lopatin, Smetana and Schöller2010). It has been found there in association with Hypericum perforatum Linnaeus (Hypericaceae), or St John’s-wort (Rheinheimer and Hassler Reference Rheinheimer and Hassler2018). The second, Psylliodes dulcamarae (Koch, 1803) (Coleoptera: Galerucinae: Alticini), is native to western Eurasia, including Europe, Anatolia, Kazakhstan, Mongolia, and eastern Siberia (Döberl Reference Döberl2010). It has primarily been associated in Europe (Rheinheimer and Hassler Reference Rheinheimer and Hassler2018) with Solanum dulcamara Linnaeus (Solanaceae). We also discuss our findings in the context of the diversity and origins of accidentally introduced Chrysomelidae in North America.
Methods
Co-authors CC and SD collected specimens during independent insect-collecting activities. CC noted that neither species matched known North American species and sent specimens to co-author HD for identification through the Agriculture and Agri-Food Canada National Identification Service, Canadian National Collection of Insects, Arachnids and Nematodes (Ottawa, Ontario, Canada). SD learned about the discovery of Cryptocephalus moraei from CC and recognised that he also had specimens of C. moraei from Québec. SD also searched near Montréal, Québec for additional specimens and further ecological associations during the summers of 2019 and 2020. Associated plants were identified visually, in some cases with supporting photographs. HD searched for specimens at three sites with Hypericum in Ottawa, Ontario, Canada during summer 2020. Specimens are deposited in the following Canadian insect collections: Claude Chantal Insect Collection (private collection), Varennes, Québec, Canada; Pierre de Tonnancour Insect Collection (private collection), Terrasse Vaudreuil, Québec, Canada; Stéphane Dumont Insect Collection (private collection), Montréal, Québec, Canada; and the Canadian National Collection of Insects Arachnids and Nematodes, Ottawa Ontario, Canada. HD and KS dissected and compared specimens to North American (White Reference White1968; Quinn Reference Quinn2017) and European (Burlini Reference Burlini1955; Doguet Reference Doguet1994; Nadein Reference Nadein2010) taxonomic literature and expert-managed Internet sites (Borowiec Reference Borowiec2010–2020) and identified the Canadian National Collection specimens, also sending images of Canadian specimens of C. moraei to J. Bezděk (Mendel University, Brno, Czech Republic) and images of P. dulcamarae to M. Gikonyo (Max Planck Institute for Chemical Ecology, Germany), who performed independent photo-based identifications.
For DNA analysis, a single leg was detached from each specimen and was placed in a well in a 96-well microplate prefilled with 10 µL of 96% ethanol. Each specimen was also photographed, and the resulting image was uploaded to the Barcode of Life Database (BOLD; Ratnasingham and Hebert Reference Ratnasingham and Hebert2007) along with the label data. The DNA extraction, polymerase chain reaction amplification, and Sanger sequencing of the cytochrome oxidase subunit 1 barcode region were performed for all specimens at the Centre for Biodiversity Genomics (University of Guelph, Guelph, Ontario, Canada), using standard protocols as outlined by Pentinsaari et al. (Reference Pentinsaari, Anderson, Borowiec, Bouchard, Brunke and Douglas2019). Primers LepF1 and LepR1 (Hebert et al. Reference Hebert, Penton, Burns, Janzen and Hallwachs2004) were used for polymerase chain reaction amplification. Sequences were obtained through unidirectional analysis. Details on the polymerase chain reaction and sequencing protocols for each specimen are provided in the public BOLD dataset information below.
Detailed collection information for each specimen, including both DNA-barcoded material and other specimen records, as well as GenBank accession numbers for the barcode sequences, are listed in the text. All sequences, details on polymerase chain reaction and sequencing primers, photographs, and full collection data for the DNA-barcoded specimens are available through a public dataset on BOLD (https://doi.org/dx.doi.org/10.5883/DS-CRYPPSYL).
The total number of adventive Chrysomelidae in North America north of Mexico has not been defined, but Klimaszewski et al. (Reference Klimaszewski, Hoebeke, Langor, Douglas, Borowiec and Hammond2020) found Canada hosted 61 species of adventive Chrysomelidae. The lack of current total estimates for North America may originate from a history of publications for Bruchinae that are separate from the remainder of Chrysomelidae and the fact that the catalogue by Riley et al. (Reference Riley, Clark and Seeno2003) did not focus primarily on adventive species. Here, we review the recent literature to assemble a list of adventive Chrysomelidae for North America.
Results and discussion
Cryptocephalus moraei
The external morphology and genitalia of Canadian specimens closely matched Burlini’s (Reference Burlini1955) taxon concept of C. moraei, as based on European specimens; J. Bezděk also confirmed our identification.
Cryptocephalus moraei was found at the following localities in Canada, Québec: Verchères, Varennes, 16.vii.2017, Claude Chantal 1 ex.; Laval, Rue St-Boniface, 45.5964, –73.6827, 6.vii.2013, swimming pool, 1 ex., S. Dumont; Laval, Boisé Papineau, 45.6078, –73.6849, 28.vi.2014, mixed meadow with Equisetum arvense–Potentilla reptans, 3 ex., S. Dumont; same except, 15.vii.2019, 6 ex. (including DNA-barcoded specimen CCDB19514_CNC_P1A10, CNC1053107; GenBank: MW564587); same except, 22.vii.2019, 5 ex. and Lotus corniculatus, 1 ex.; Montréal, Parc Zotique-Racicot, 45.5416, –73.6831, 24.vii.2015, Plantago lanceolata, 1 ex., S. Dumont; same except, 45.5426, –73.6903, 11.vii.2018, Cirsium arvense, 1 ex.; same except 45.5432, –73.6916, 17.vii.2019, Medicago lupulina, 4 ex. (including DNA-barcoded specimen CCDB19514_CNC_P1A9, CNC1053106; GenBank: MW564586); same except, 18.vii.2019, 1 ex. + Hypericum perforatum, 1 ex.; same except, 24.vii.2019, Medicago lupulina, 6 ex.; same except, 30.vii.2019, 3 ex.; same except, 1.viii.2019, 1 ex; same except 14.vi.2020, 2 ex.; same except 19.vi.2020, 1 ex. + Montréal, Bois-de-Boulogne train station, 45.5407, –73.6754, Potentilla reptans, 6 ex.; Montréal, Parc Zotique-Racicot, 45.5432, –73.6916, 28.vi.2020, Medicago lupulina, 13 ex.; same except, 5.vii.2020, 6 ex., S. Dumont with P. de Tonnancour; same except, 18.vii.2020, 7 ex., S. Dumont; same except, 22.vii.2020, 2 ex.; same except, 24.vii.2020, 1 ex.; same except, 11.viii.2020, 1 ex.
Specimens were swept from Equisetum Linnaeus (Equisetaceae) and Potentilla Linnaeus (Rosacea) stands and from individual plants of Hypericum Linnaeus (Hypericaceae), Lotus Linnaeus (Fabaceae), Medicago Linnaeus (Fabaceae) (Fig. 1D), Plantago Linnaeus (Plantaginaceae), and Trifolium Linnaeus (Fabaceae) growing in dry–mesic meadows on disturbed rocky subsoil (Fig. 1), with Cryptocephalus venustus (Fabricius, 1787) also present. No C. moraei specimens were found in wet-soil parts of one of the same sites. The earliest collection year for C. moraei was 2013 in Laval, Québec. Specimens from the Montréal, Québec sites were collected between 14 June and 11 August 2020. Searches were not conducted during 3–13 August 2019 or between 31 July and 7 August 2020. Searches by SD on 14, 18, and 31 August 2019, on 17, 20, 24, and 30 May 2020, and on 16 and 19 August 2020 yielded no specimens. The distances between collection site clusters were 36 and seven kilometres in the Montréal area, including the south shore of the St. Lawrence River and the two largest islands of the Hochelaga Archipelago. Collection of 74 specimens in three areas over seven years suggests that C. moraei is established at multiple sites in Canada. Searches at three similar sites in central Ottawa, Ontario, Canada yielded no specimens. No wider survey has been conducted to determine the full range of this species in North America.

Fig. 1. Habitat and resting plants used by Cryptocephalus moraei from Québec, Canada: A, Boisé Papineau, Laval, Québec, Canada; B, Parc Zotique-Racicot, Montréal, Québec; C, on leaf (possibly of Pastinaca sativa Linnaeus (Apiacieae)); and D, under leaf of Medicago lupulina.
Cryptocephalus moraei can be distinguished from other North American and European Cryptocephalus by body length, 3–5 mm, with nonmetallic and mainly black dorsal and ventral colouration (Fig. 2A, B), body glabrous dorsally; head with pale markings on frons (X-shaped in male, Fig. 2C; female with two red longitudinal lines in most); pronotum pale at anterior edge and hind angles in male (Fig. 2A, C, D) and in some females, pronotal lateral carina not visible in dorsal view, punctures not forming striae; elytra striate with pale patches near humeri and apices (Fig. 2A, B), humeral pale patches not reaching elytral base or suture; legs with ventral side of profemora pale (Fig. 2A), tibiae and tarsi pale (modified from Mohr Reference Mohr1966). The aedeagal shape (Fig. 3A–C) also distinguishes this from previously illustrated North American Cryptocephalus species (White Reference White1968). The prosternum also has pale peg-like postcoxal projections (Fig. 2D) not observed in other North American Cryptocephalinae. Females can be distinguished from males by dark anterior edge of the pronotum and a mesal depression on ventrite 5. The eastern North American Cryptocephalus quadruplex Newman, 1841 is similar, but pale markings are absent from the head and pronotum, and most specimens have the humeral pale spot reaching the elytral base.
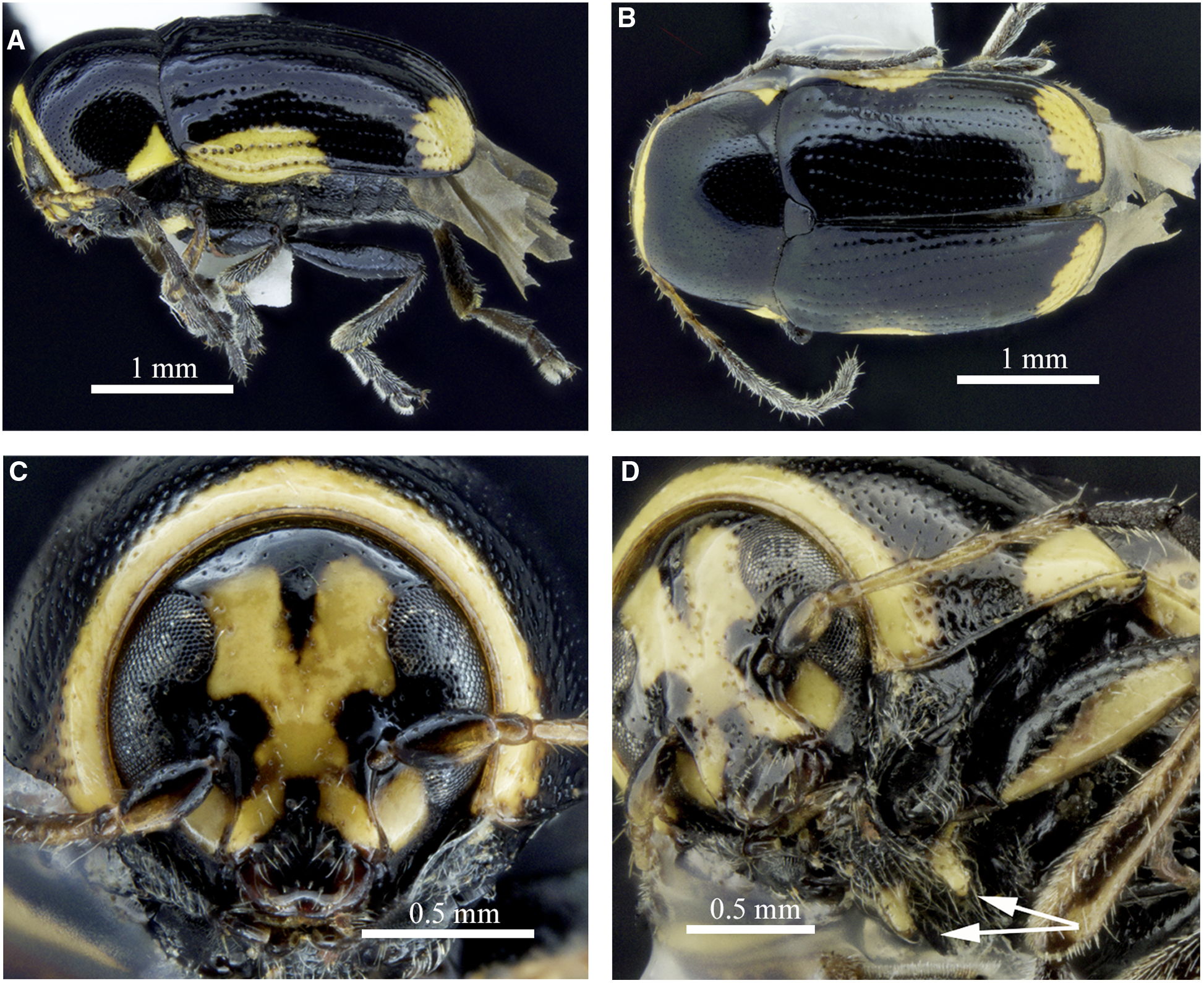
Fig. 2. Morphology of a male of Cryptocephalus moraei from Québec, Canada: A, lateral habitus; B, dorsal habitus; C, anterior view of head; and D, ventrolateral view of prosternum with arrows to postcoxal projections.

Fig. 3. Genitalia of Cryptocephalus moraei from Québec, Canada: A, aedeagus, dorsal view; B, aedeagus, lateral view; C, aedeagus apex in apico-dorsal view; and D, spermatheca.
Although C. moraei is primarily associated with Hypericum perforatum Linnaeus (Hypericaceae) (also called St John’s-wort; Rheinheimer and Hassler Reference Rheinheimer and Hassler2018), only one Canadian adult specimen was also found on H. perforatum. Hypericum perforatum is an adventive weed in North America that has been subject to biological control efforts. However, C. moraei is not expected to substantially damage Hypericum plants because its larvae consume senescent leaves (Rheinheimer and Hassler Reference Rheinheimer and Hassler2018).
Analysis of the two DNA-barcoded Canadian specimens of C. moraei through the BOLD Identification Engine resulted in a 98.78% match with Cryptocephalus moraei. The BOLD Barcode Index Number (Ratnasingham and Hebert Reference Ratnasingham and Hebert2013) cluster assignments were not available for our specimens at the time of publication. Comparison of sequences of our specimens to publicly available specimens from European barcoding initiatives (Pentinsaari et al. Reference Pentinsaari, Hebert and Mutanen2014; Hendrich et al. Reference Hendrich, Morinière, Haszprunar, Hebert, Hausmann, Köhler and Balke2015) indicated sequence divergences of 1.2–4.3% among 26 specimens from barcode index numbers ACE2080 and AAN3893. The barcodes from the Canadian specimens were closer to barcode index number ACE280, with specimens from Finland and Estonia, than to barcode index number AAN3893, with specimens from Germany, Italy, Spain, France, Austria, Netherlands, and Norway. Clustering of the Canadian specimens within European sequences of C. moraei is consistent with our morphological identification.
Psylliodes dulcamarae
The external morphology and male genitalia of Canadian specimens closely matched Nadein’s (Reference Nadein2010) taxon concept of P. dulcamarae, as based on European specimens; M. Gikonyo also confirmed our identification. We identified Psylliodes specimens as P. dulcamarae using Nadein (Reference Nadein2010) and Doguet (Reference Doguet1994). They also matched Canadian National Collection specimens identified by Doguet in body shape, frons morphology and colour pattern, punctation of the pronotum and elytra, aedeagus shape, and spermatheca shape. The spermatheca matches the image published by Doguet (Reference Doguet1994, Fig. 223i) in the shapes of the pump and duct; however, the receptacle is about one-third narrower than in the specimen illustrated. This suggests that there may be undocumented spermathecal receptacle-shape variability in the native range of this species.
Analysis of the DNA-barcoded Canadian specimen of P. dulcamarae through the BOLD Identification Engine resulted in a 99.69% match with Psylliodes dulcamarae. The BOLD barcode index number assignments were not available for our specimens at the time of publication.
Comparison of sequences of our specimen to specimens from the German Barcode of Life project (Hendrich et al. Reference Hendrich, Morinière, Haszprunar, Hebert, Hausmann, Köhler and Balke2015) indicated sequence divergences of 0.3–0.9% among the eight specimens with publicly available data compared. This result is consistent with morphological identification of P. dulcamarae.
One author (CC) found P. dulcamarae at the following localities in Canada, Québec: Verchères, Varennes, 9.vi.2010, 1 ex.; 15.v.2012, 2 ex.; 27.vi.2013, 1 ex.; 11.v.2014, 1 ex.; 21.v.2014, 1 ex.; 23.v.2014, 2 ex.; 11.vi.2014, 4 ex.; 30.vi.2014, 1 ex.; 8.vii.2014, 1 ex.; 30.vii.2014, 2 ex.; 15.v.2015, 1 ex. (DNA-barcoded specimen CCDB19514_CNC_P1A11, CNC1053108; GenBank: MW564584); 18.v.2015, 1 ex.; 1.vi.2015, 1 ex.; 7.vi.2015, 2 ex.; 2.vii.2015, 1 ex.; 24.v.2018, 1 ex.; Boucherville, woodland edge by industrial area, 45.55, –73.42, 25.v.2012, 3 ex.; same data except 19.v.2014, 1 ex. Collection of 27 specimens from two sites on the south shore of the St. Lawrence River, Québec separated by 25 km over eight years suggests that populations of P. dulcamarae are established in Canada. No survey has been conducted to determine the full range of this species in North America.
Genus Psylliodes requires taxonomic revision; however, P. dulcamarae can be distinguished from other eastern North American Psylliodes by the following combination of characters: metallic-blue colouration and uniformly dark legs (Fig. 4A, B). Its aedeagus and female genitalia also appear unique among described North American species (Fig. 4D–G).

Fig. 4. Morphology of Psylliodes dulcamarae from Québec, Canada: A, male dorsal habitus; B, male lateral habitus; C, male tarsal claw; D, vaginal palpi; E, aedeagus, dorsal view; F, aedeagus, lateral view; and G, spermatheca.
Psylliodes dulcamarae has primarily been associated in Europe with Solanum dulcamara (Rheinheimer and Hassler Reference Rheinheimer and Hassler2018), a species with red berries that are probably toxic to humans in large quantities (Alexander et al. Reference Alexander, Forbes and Hawkins1948; Hornfeldt and Collins Reference Hornfeldt and Collins1990) and possibly also to livestock (Waggy Reference Waggy2009). Although Solanum dulcamara is not native to North America, it has apparently not been a subject of biological control research efforts (Mason and Huber Reference Mason and Huber2002; Mason and Gillespie Reference Mason and Gillespie2013). It has perhaps not received biological control research attention because of its low stand density in nature and because it is congeneric with potato, tomato, and peppers. Its relatedness to cultivated vegetables presents a risk that herbivorous biological control agents could also attack crops. However, to date, P. dulcamarae has not been found associated with important damage to crop species. This species may benefit agriculture, human safety, and native plant communities in North America by harming S. dulcamara plants.
Adventive species biology
Interestingly, P. dulcamarae is the second pest of Solanum dulcamara to be discovered recently in North America. Deczynski (Reference Deczynski2014, Reference Deczynski2019) recently reported Epitrix pubescens (Koch, 1803) (Coleoptera: Chrysomelidae) from the northeastern United States of America and eastern Canada, with specimens known from as early as 1975. It seems likely that these two flea beetle species that feed on the same host may have come to North America along the same trade pathway. One possible pathway for such introductions is importation of woody plants with soil. For example, some populations of Elateridae (Coleoptera) that were detected as larvae in shipments of woody plants shipped with soil during 1960–1965 have only recently been detected as adventive in North American cities (Douglas Reference Douglas2011). Many flea beetle larvae also develop around the roots of weedy hosts, which are likely to also have been shipped to North America in soil with woody ornamental plants.
Cryptocephalus moraei is the first Palearctic species of Cryptocephalinae introduced elsewhere in the world (although the Nearctic Diachus auratus Fabricius, 1801 now has a cosmopolitan distribution; Reid Reference Reid1988; Regalin and Medvedev Reference Regalin and Medvedev2010; Schöller et al. Reference Schöller, Löbl and Lopatin2010). Cryptocephalinae with larvae mainly inhabiting leaf litter are probably less prone to accidental transport by humans via plant tissue than are herbivores feeding on or in growing plant tissues. Finding such an introduction in a northeastern North American city is also consistent with the former practice of importing European plants in soil to North America.
Origins of adventive Chrysomelidae in North America
Before these findings, Canada and the United States of America were together known to host 66–76 species of adventive Chrysomelidae (Table 1). This estimate is based on 44 adventive and possibly adventive Chrysomelidae reported from North America by Riley et al. (Reference Riley, Clark and Seeno2003). Riley et al. (Reference Riley, Clark and Seeno2003) also reported three additional nonnative species of uncertain population-establishment status in North America. Kingsolver (Reference Kingsolver2004) added 14 adventive Bruchinae and two North American Bruchinae of possible adventive origins to these numbers. Klimaszewski et al. (Reference Klimaszewski, Hoebeke, Langor, Douglas, Borowiec and Hammond2020) reviewed recent records of adventive Chrysomelidae in Canada (including recent additions by Pentinsaari et al. Reference Pentinsaari, Anderson, Borowiec, Bouchard, Brunke and Douglas2019), finding seven additional species for the North American fauna. Five additional species are reported from the United States of America in four other publications (see Table 1). These sources, together with our findings, indicate that 68–78 species of adventive Chrysomelidae are known from Canada and the United States of America. Of these, 51–59 are known from Canada, and 56–66 are known from the United States of America. These numbers include 21 species intentionally introduced as biological control agents. The numbers also capture uncertainty about the presence of six nonnative species and about nonnative status of four recorded species. Unverified Internet reports suggest that at least two additional species of Palaearctic Chrysomelidae occur in the United States of America. It is also likely that some species reported from only the United States of America (northern parts) or only Canada also occur in the other country because detection surveys have not been done.
Table 1. Adventive Chrysomelidae reported from Canada and the United States of America. Unless noted otherwise, all are established, accidentally introduced nonnative species that are present in both countries. References cited are numbered as follows: (1) Riley et al. Reference Riley, Clark and Seeno2003; (2) Clark et al. Reference Clark, LeDoux, Seeno, Riley, Gilbert and Sullivan2004; (3) Kingsolver Reference Kingsolver2004; (4) Hoebeke et al. Reference Hoebeke, Wheeler, Kingsolver and Stephan2009; (5) Overholt et al. Reference Overholt, Diaz, Hibbard, Roda, Amalin and Fox2009; (6) Tracy and Robbins Reference Tracy and Robbins2009; (7) Overholt et al. Reference Overholt, Rayamajhi, Rohrig, Hight, Dray and Lake2016; and (8) Klimaszewski et al. Reference Klimaszewski, Hoebeke, Langor, Douglas, Borowiec and Hammond2020.

Except for seed-feeding Bruchinae, nearly all accidentally introduced species both originated in Europe and occur in Canada (Klimaszewski et al. Reference Klimaszewski, Hoebeke, Langor, Douglas, Borowiec and Hammond2020). The exceptions to this are two species that feed on Australian Eucalyptus L’Héritier de Brutelle (Myrtaceae) that are known from southwestern United States of America and which may have been introduced intentionally (Paine et al. Reference Paine, Millar and Daane2010). This abundance of European adventive species indicates that although Asia is the source of some important recent introductions of beetles in other families, most newly discovered adventive Chrysomelidae species in North America continue to originate from Europe. This is consistent with the hypothesis that recent discoveries of introduced species represent expanding populations of species established 50–60 years earlier via former trade-related introduction pathways carrying larger numbers of soil-dwelling insects. Findings of accidentally introduced species in Canada but not in adjacent areas of the United States of America suggest that the fauna of the northeastern United States of America may include several undocumented adventive chrysomeloid species.
Acknowledgements
The authors thank J. Bezděk (Mendel University, Brno, Czech Republic) for confirming identification of C. moraei and M. Gikonyo (Max Planck Institute for Chemical Ecology, Germany) for confirming identifications of P. dulcamarae. Thanks to J.-F. Landry (Agriculture and Agri-Food Canada) for methodological advice. The authors extend their thanks to M. Pentinsaari (University of Guelph), E.G. Riley (Texas A&M University), and an anonymous reviewer for comments that helped improve the manuscript.








