Introduction
The bark beetles (Coleoptera: Curculionidae: Scolytinae) have long been recognised as key ecological denizens of Canadian forests. These disturbance agents provide a wide array of ecological services; functioning in nutrient cycling, aiding forest succession, mediating watershed hydrology, and affecting aesthetics, recreation, and property values (Safranyik et al. Reference Safranyik, Shrimpton and Whitney1974; Richmond Reference Richmond1986). At biome-level scales, recent extensive outbreaks have had an impact on biogeophysical processes such as carbon cycling and sequestration (Kurz et al. Reference Kurz, Dymond, Stinson, Rampley, Neilson and Carroll2008; Hicke et al. Reference Hicke, Allen, Desai, Dietze, Hall and Hogg2012). Considerations of the multifaceted impacts of bark beetles at multiple scales have broadened considerably since the beginning of the 20th century, when bark beetles were viewed almost entirely as an economic nuisance, and harbinger of potentially dangerous forest fires (Brown Reference Brown1940).
Through that lens, Mr. J.M. Swaine, the assistant entomologist in charge of forest insect investigations (Fig. 1), set out to compile for the Dominion of Canada a treatise on the “True bark-beetles” during the years of World War I (Swaine Reference Swaine1918). Swaine fastidiously gathered accounts of bark beetle ecologies and methods for control, with descriptions of several new species. Calling Swaine one of “the most inspiring men [with whom] I have ever been associated”, Mr. Hector A. Richmond, a pioneering forest entomologist who worked with Swaine in western Canada, noted that following the course of Swaine’s activities in the woods was like “tracking the wanderings of an old bear. Every rotten stump or log was torn apart in his quest for various beetles while I, as an observer, would follow and gather up his lost or misplaced tools and equipment” (Richmond Reference Richmond1986). Swaine’s enthusiastic investigations and consultations with other early forest professionals were very fruitful, however. Swaine’s excursions culminated in the classic text of 1918, “Canadian bark-beetles: a preliminary classification, with an account of the habits and means of control” that was promptly submitted by Dominion Entomologist and Consulting Zoologist C. Gordon Hewitt for approval to the Honourable Minister of Agriculture. It was hoped that the knowledge in the book would be used to “protect extensive areas that are threatened by the attacks of bark-beetles”, which Swaine declared the “chief insect enemies of our coniferous forests” (Swaine Reference Swaine1918).

Fig. 1 J.M. Swaine, Assistant Entomologist in charge of Forest Insect Investigations, circa 1919. Reprinted from Johnstone (Reference Johnstone1991).
Swaine’s treatise covered 100 species of bark beetles, but focussed on those with potential to cause economic loss, as “the protection and correct utilization of our timber resources is of greater importance than ever from a national and imperial standpoint”. Perhaps the most notable achievement of Swaine’s first text was the inclusion then of every Dendroctonus Erichson species known to exist within Canada today! In an era devoid of airline, internet, cellular, satellite, and social media coverage – indeed, in an era where the two most critical tools to a forest entomologist were but “a tent and an axe” (Richmond Reference Richmond1986, quoting Swaine) – we may easily take for granted such a historic feat. Swaine also included several other important economic species in his tome, such as western balsam bark beetle Dryocetes confusus Swaine and the four-eyed spruce beetle Polygraphus rufipennis (Kirby).
Reviewing and/or synthesising a century of bark beetle population dynamics in Canada from Swaine’s early explorations to the present requires three important admissions. First, much worthy material has undoubtedly been omitted. Certain exclusions are due to strategic choices necessitated by writing a journal article instead of a book. The reader hoping for coverage of past theories of the roles of lance flies (Diptera: Lonchaeidae) in the termination of outbreaks of mountain pine beetle Dendroctonus ponderosae Hopkins may be sorely disappointed (Richmond Reference Richmond1986), although more egregious eliminations exist. More disconcertingly, other omissions are due to inadvertent losses from the national record. During the Depression era of the 1930s, for example, federal bark beetle ecologists in western Canada conducted extensive studies on the reproductive biology of mountain pine beetle, including work on host selection and the roles of competitors, parasites, and vertebrate predators in regulating populations. Unfortunately, results of these investigations were never published due to budget reductions and federal discouragement of publication (Richmond Reference Richmond1986). Our second admission is that we are forest entomologists and not historians. Trained practitioners of the latter discipline may have exercised different editorial choices. Third, similar to Swaine, we focus largely on tree-killing species.
That said, in this work we first honour the heritage of Swaine by summarising the foundational aspects of his early textbook common to many bark beetles: their “general habits”, classifications of behaviour, presumed causes of population increase, and tactics to mitigate their effects. Broad advances in the study of the ecology and population dynamics of bark beetles are highlighted. Swaine also provided notes on morphology, hosts, gallery patterns, and damage for a number of economically important species. A full comparison is beyond the scope of the current work; instead, we summarise what is known today regarding the life histories, population dynamics, and management of D. rufipennis Kirby, D. pseudotsugae Hopkins, and D. simplex LeConte. Because the population dynamics of D. ponderosae have been well described in recent work (Safranyik and Carroll Reference Safranyik and Carroll2006), we restrict our treatment of mountain pine beetle to brief comments on range expansion. We discuss how another aspect of global change, invasive species, has altered the outlook of some of the bark beetles mentioned by Swaine. Finally, we close with two adages from Swaine and Richmond that hold true today in the face of a changing world.
Bark beetles and the procurement of host trees
Bark beetles are cryptic species that feed and reproduce within the phloem and sometimes the outer bark tissues of trees. Swaine termed these insects the “true bark-beetles”, excluding those bark beetles that putatively breed in plants of the gourd family; behaviour he jettisoned as “aberrant habits much more common in tropical countries than with us” (Swaine Reference Swaine1918). Bark beetles derive their common name from their life history habits underneath the bark. The subcortical patterns of gallery etchings during reproduction, in tandem with host tree information, are frequently used to identify species. Swaine included plates illustrating gallery formations across a range of taxa before diagnostic illustrations of the insects themselves. Swaine’s simple techniques of observing mating and gallery construction behaviour among bark beetles in sandwiches of phloem and bark held between glass plates is still widely used today (Kim and Miller Reference Kim and Miller1981; Taylor et al. Reference Taylor, Hayes, Roton and Moser1992; Dodds et al. Reference Dodds, Graber and Stephen2001; Aukema and Raffa Reference Aukema and Raffa2004).
In a given species of bark beetle, typically one sex is the host-selecting sex (Kirkendall Reference Kirkendall1983). Pioneering adults will chew through the bark into the phloem tissues before being joined quickly by large numbers of conspecifics. Following mating under the bark, females will begin constructing ovipositional galleries along which eggs are laid. After a feeding period, the larvae pupate at the terminal end of the feeding gallery. New adult beetles emerge by chewing through the bark. In some species, mating occurs under the bark before emerging to procure new hosts (Bleiker et al. Reference Bleiker, Heron, Braithwaite and Smith2013). The time spent by adults outside a host is typically very short; only days, as the insects seek new host material (Raffa Reference Raffa2001).
Although the preceding generalised description of bark beetle natural history has not changed substantially in the past century, great strides have been made in understanding the processes by which beetles procure host trees. These discoveries relate to three fields of study: plant–insect interactions, pheromone ecology, and microbiology. As each of these topics is treated elsewhere in this issue, the contributions of each are summarised here in brief.
First, there is now a greater understanding of how bark beetles interact with host tree defences, and how those defences may inhibit or synergise colonisation activities (Raffa and Berryman Reference Raffa and Berryman1983). Swaine’s understanding of insect–host interactions 100 years ago was that bark beetles were primarily confined to “dying bark” and only entered live trees during outbreaks. Today, we have a much broader understanding of the feeding niches of bark beetles within hosts (Ayres et al. Reference Ayres, Ayres, Abrahamson and Teale2001), as well as how the suitability and susceptibility of breeding substrate changes with plant defence dynamics (Boone et al. Reference Boone, Aukema, Bohlmann, Carroll and Raffa2011). Beetles seeking to colonise live trees are frequently presented with increased concentrations of resin that pose a chemical and physical barrier to host entry. The tree’s defensive response can include rapid biosynthesis of several allelochemicals such as monoterpenes, resin acids, and phenolics in addition to wound compartmentalisation (Huber et al. Reference Huber, Ralph and Bohlmann2004). Our understanding of plant–insect interactions continues to advance rapidly as novel tools at the suborganismal level add to our understanding of mechanisms of both induction of plant defence and detoxification of plant compounds by the herbivores (Bohlmann et al. Reference Bohlmann, Gershenzon and Aubourg2000; Franceschi et al. Reference Franceschi, Krokene, Christiansen and Krekling2005).
Second, it is now known that pheromones mediate interactions between beetles and the host trees as colonisation is occurring (Borden Reference Borden1989). Aggregation pheromones are especially critical to host-procurement for “aggressive” species of bark beetles (discussed below). Pheromones serve a diversity of functions, such as attracting conspecifics via short-range and long-range chemical cues. Pheromones may also signal prey availability to “eavesdropping” natural enemies (kairomones), indicate habitat occupancy to interspecific competitors (allomones), and deflect conspecifics into proximal, non-colonised host material through anti-aggregation pheromones (Borden Reference Borden1989; Raffa Reference Raffa2001). Moreover, many pheromones are synergised by host compounds (Erbilgin et al. Reference Erbilgin, Krokene, Kamme and Christiansen2007).
Third, we are gaining an ever-deeper understanding of the roles of microbes and associated organisms in the colonisation of host trees by bark beetles (Six Reference Six2013). In Swaine’s book, fungi were relegated to saprophobes that destroyed the bark when conditions were too wet, rendering it unsuitable for food. Some fungi, particularly those associated with ambrosia beetles, were thought to provide nutrition (ambrosia beetles were not discussed further, as these insects were deemed not “true bark beetles”, as some, living outside of trees, were known to “cut their tunnels in the staves of wine casks”). Today, beetle-vectored fungi are recognised to fulfil diverse roles in the ecology of bark beetles, such as providing nutrition to developing broods, but also competing against pathogenic fungi, detoxifying and disrupting plant defences, detoxifying host compounds, and producing semiochemical compounds with components identical to those found in bark beetle pheromones (Boone et al. Reference Boone, Six, Zheng and Raffa2008; Klepzig et al. Reference Klepzig, Adams, Handelsman and Raffa2009; Lieutier et al. Reference Lieutier, Yart and Salle2009; DiGuistini et al. Reference DiGuistini, Wang, Liao, Taylor, Tanguay and Feau2011). Bacteria were not mentioned in Swaine’s treatise, although we now know that bacteria and fungi have complex relationships and our understandings of them are in their infancy (Cardoza et al. Reference Cardoza, Klepzig and Raffa2006). Similarly, mites (Acari) are now recognised as important vectors of fungi and bacteria, with mutualistic and antagonistic interactions structuring and influencing community and population dynamics (Hofstetter and Moser Reference Hofstetter and Moser2014).
Classifications of bark beetles
Swaine’s early work set the stage by which we now categorise the eruptive behaviour of bark beetles, recognising that there is a continuum of the tendency to attack and kill live and vigorous versus stressed and moribund trees. Swaine termed these behaviours indicative of “primary” versus “secondary” “enemies”. The “primary” and “secondary” designations found widespread use in the ecological literature for the next several decades until being replaced with “aggressive” and “nonaggressive”. These latter terms avoid unintended connotations regarding sequence of attack, as some less aggressive beetles such as pine engravers Ips pini (Say) may become principal tree-killing agents on occasions when populations are high (Kegley et al. Reference Kegley, Livingston and Gibson1997).
Approximately 98% of bark beetles are “non-aggressive” species that colonise cones, twigs, or branches, or dead or stressed trees (Lindgren and Raffa Reference Lindgren and Raffa2013). Such trees are easier to colonise because they lack the induced defensive response of live hosts, but present a trade-off in increased competition from other saprophages, decreased nutritional value of substrate for developing brood, and are ephemeral in space and time. In contrast, live trees present a nutritionally superior resource, but can mount vigorous physical and chemical defensive responses to attacking beetles (Raffa and Berryman Reference Raffa and Berryman1983). Several “aggressive” species of bark beetles thus use aggregation pheromones to attract large numbers of conspecifics to the host and collectively overwhelm host defences. Outbreaks of tree-killing bark beetles can continue for years, unabated, until either the host supply is exhausted or environmental conditions return insect populations to an endemic state (Raffa et al. Reference Raffa, Aukema, Bentz, Carroll, Hicke and Turner2008).
Swaine did describe a third category of bark beetles. “Neutral” species putatively bred only in dying bark and were not known to cause any injury to living trees. Most of these species were found in hardwoods, such as the elm bark beetle, Hylurgopinus rufipes (Eichhoff). We are not aware of any “neutral” classifications applied to bark beetles after the publication of Swaine’s work, as most “neutral” bark beetle species would seem to share colonisation traits with non-aggressive species.
Swaine appeared well aware of the current limitations of bark beetle taxonomy at the time of his work. A.D. Hopkins, considered the father of forest entomology in North America, had completed an extensive study of world species (Hopkins Reference Hopkins1909a) that Swaine deemed “an exceedingly valuable contribution”. Swaine was reluctant, however, to agree with the new terminology of “Scolytidae”, relegating it instead to subfamily “Scolytinae” and retaining a more acceptable family name “Ipidae”. Disagreements about the status of Scolytidae as a family within Order Coleoptera are still prevalent today (e.g., Bright Reference Bright2014). Swaine submitted that a final acceptable arrangement of the Ipidae could be made only after a more complete study of world species in the future. Indeed, Swaine lamented the “dearth of biological papers on North American bark beetles”, submitting that “time spent upon even the species of apparently minor economic importance may give decidedly practical results”. Swaine cited four genera of “secondary” bark beetles, for example, as being beneficial to trees by acting to kill lower branches such that “cleaner trunks, and thus better logs” could be produced (genera Polygraphus Erichson, Eccoptogaster Herbst, Pityogenes Bedel, and Pityophthorus Eichhoff; Swaine Reference Swaine1918).
Swaine correctly surmised that most of our understanding of taxonomic breadth of bark beetles would come from the non-aggressive species, as aggressive species were becoming well known. As could be expected, there was a heavy bias on the “primary enemies” as they attacked “perfectly sound trees” causing the “chief” or “primary” injury. In total, Swaine described exactly 100 species of bark beetles in the classification section of his book (Swaine Reference Swaine1918). By 1976, when Bright published “The bark beetles of Canada and Alaska”, the list had grown to 214 species of Scolytinae (Bright Reference Bright1976). Even in doubling the list, however, Bright praised Swaine’s effort as “one of the finest works available on the bark beetles of North America”.
Factors mediating bark beetle population dynamics
While mortality to trees could be profound in the early 20th century, elucidating the factors affecting the rise and fall of bark beetle populations was still in its infancy. Swaine postulated that the moisture content of the air in the ovipositional tunnels, temperature of the air and bark, and sunlight were the three most important factors affecting the population dynamics of bark beetles (Swaine Reference Swaine1918; Anderson Reference Anderson1948). The prevailing dogma held that bark beetle populations were simply favoured in conditions that were qualitatively described as warm and moderately dry, and not too wet or cold. These understandings were not developed so much from studies of stand resistance or tree growth rates but rather detailed studies of developing broods of bark beetles in bark sandwich assays. It was thought, for example, that beetles would work to plug excess ventilation holes quickly to preserve humidity levels in the ovipositional galleries. Sunlight warmed the brood environment, but could also be detrimental if desiccation of the bark occurred (Swaine Reference Swaine1918).
Although many early ideas of conditions that fostered beetle success focussed on subcortical environs within a tree, attention broadened to focus on stand conditions in the ensuing decades. This switch was likely precipitated in part by the association of bark beetle outbreaks with periods of drought as longer time series of data on outbreaks became available (Blackman Reference Blackman1931; Beal Reference Beal1943). Diminished growth rates were considered to be a simple metric of moisture stress, decreasing vigour, and/or increased risk of bark beetle outbreaks (Hopping and Mathers Reference Hopping and Mathers1945; Hopping and Beal Reference Hopping and Beal1948; Safranyik et al. Reference Safranyik, Shrimpton and Whitney1975). By the middle of the 20th century, foresters had noted that sufficient soil moisture permitted conifers to drown attacking beetles with excessive resin flow (Prebble Reference Prebble1951).
While the frameworks for population dynamics involving “density-dependent” and “density-independent” factors had not yet been formally developed by Andrewartha and Birch (Reference Andrewartha and Birch1954) and Nicholson (Reference Nicholson1954), Swaine took pains to make clear that regardless of conditions all increases of bark beetles took place against a template of host availability. Other potentially density-dependent natural controls, such as parasitoids, mites, birds, and fungi received mention in his text, but were dismissed as of minor importance, or restricted to regulating populations of non-aggressive species. While we continue to recognise the importance of availability of breeding substrate as a fundamental density-dependent factor regulating bark beetle population dynamics (Prebble Reference Prebble1951; Økland and Bjørnstad Reference Økland and Bjørnstad2006; MacQuarrie and Cooke Reference MacQuarrie and Cooke2011), the definition of what constitutes a suitable host has changed substantially over the past century.
Swaine suggested that “all our bark beetles have, normally, a preference for dying bark”. Hence, any condition that presented an abundance of dying bark was a recipe for increasing bark beetle populations. Critical problem areas included logging operations, settlers’ clearings, and cuttings for firewood and trail-making. Swaine declared injured and “slightly burned” trees to be particularly dangerous sources of beetles, although “those [trees] thoroughly charred from base to top may be disregarded”. Logically then, Swaine declared that the key principal of control was to reduce the amount of suitable breeding substrate to “the normal amount of dying bark to be found in the woods [sufficing] for breeding purposes”. Burning of slash after logging operations was crucial, although acceptable clean-up could also be accomplished by sawing or debarking and burning of the wood and bark in winter, and/or occasionally creating and destroying trap trees. Combinations of methods could be used to ensure completion during the late fall, winter, and early spring before emergence of adults in later spring and summer. Whether contending with “windfalls, snow-breaks, [or] flood injuries”, the singular goal was simply to “have the dying timber utilized or destroyed before it can give forth its crop of destructive beetles” (Swaine Reference Swaine1918).
In his writings on feeding preferences, Swaine proposed an early formulation of a principal that would later become synonymous with the name A.D. Hopkins. It was obvious from discussion of the suitability of family names of Ipidae versus Scolytidae that Swaine was aware of and had deep appreciation for the work of Hopkins (Reference Hopkins1909a). Hopkins’ host selection principal states that adult insects will breed in host species from which they themselves originated as larvae (Hopkins Reference Hopkins1916; Barron Reference Barron2001). Swaine invoked a less stringent framework – devoid of host species preference – but agreed that adult behaviour may mirror prior larval experience in describing the formation of outbreaks. While Swaine stated that all bark beetles were predisposed to dying bark, he asserted that “once they taste green timber they will return to it… Broods of our most injurious species which have bred in an epidemic outbreak in green trees have apparently a decided tendency towards green timber”. As we have come to recognise more recently, eruptions among aggressive species of bark beetles dominated by positive feedbacks (Raffa et al. Reference Raffa, Aukema, Bentz, Carroll, Hicke and Turner2008) are associated with density-dependent shifts in host selection from moribund to healthy trees (Raffa et al. Reference Raffa, Aukema, Bentz, Carroll, Hicke and Turner2008; Boone et al. Reference Boone, Aukema, Bohlmann, Carroll and Raffa2011).
Given this behaviour, Swaine thus offered some rules of thumb for management, such as a requirement to remove “three-quarters of green-attacked trees over a winter, with mop up the following year” as such situations occurred. Some 10 years after publication of his textbook, in fact, Swaine declared such methods so reliable in halting populations of mountain pine beetle transitioning to the outbreak stage that “we may confidently look forward to the time when these destructive outbreaks will be prevented throughout the commercial lodgepole pine (Pinus contorta Douglas ex Loudon; Pinaceae) areas of British Columbia and Alberta” (Swaine Reference Swaine1929). Although Swaine underestimated the resiliency of native pine ecosystems, more nuanced, quantitative population-based frameworks for management of species such as the mountain pine beetle can, when executed properly, yield effective results (Carroll et al. Reference Carroll, Shore and Safranyik2006; Fettig et al. Reference Fettig, Klepzig, Billings, Munson, Nebeker, Negrón and Nowak2007).
Our understanding of factors that influence changes in bark beetle abundance through space and time has developed considerably from the uniform qualitative descriptions that Swaine provided. Similarly, our understanding of life histories has grown considerably. Swaine postulated that broods of the “Ipidae” (i.e., bark beetles) could develop at different rates at different altitudes and latitudes, asserting that a species of bark beetle that is “single-brooded” in northern Canada may have two broods per year in the middle or southern United States of America. We now recognise this supposition as an early formulation of Hopkins’ bioclimatic law that was being developed at approximately the same time at the Agricultural Experiment Station in West Virginia, United States of America (Hopkins Reference Hopkins1920, Reference Hopkins1938). In the ensuing 100 years, we have developed a much greater appreciation of how temperature can affect shifts in insect voltinism, from altering developmental thresholds to identifying stages of obligate diapause during insect development (Bentz et al. Reference Bentz, Logan and Amman1991; Bale et al. Reference Bale, Masters, Hodkinson, Awmack, Bezemer and Brown2002). Temperature-induced shifts in voltinism can be very important to population dynamics of tree-killing Dendroctonus bark beetles (Hansen and Bentz Reference Hansen and Bentz2003), but detailed studies on their mechanisms are still not complete (Powell et al. Reference Powell, Jenkins, Logan and Bentz2000; McKee. Reference McKee2015).
Tree-killing Dendroctonus species
Here, we describe the life histories and population dynamics of four species of tree-killing Dendroctonus bark beetles found in Canada. A century ago, Swaine singled out the spruce beetle D. rufipennis as responsible for killing “enormous quantities of spruce” on the eastern seaboard, particularly in Maine and south western New Brunswick (Swaine Reference Swaine1918). This insect is an aggressive species: its population dynamics are dominated by positive feedback when at outbreak levels, enabling it to exert biome-level influences that can last more than a decade (Berg et al. Reference Berg, Henry, Fastie, Volder and Matsuoka2006; Raffa et al. Reference Raffa, Aukema, Bentz, Carroll, Hicke and Turner2008). In contrast, eruptions of the Douglas-fir beetle, D. pseudotsugae, do not typically last more than two to four years as increasing stand resistance limits tree-killing activity as the least vigorous trees are culled from the population (Schmitz and Gibson Reference Schmitz and Gibson1996; Garrison-Johnston et al. Reference Garrison-Johnston, Moore, Cook and Niehoff2003). The eastern larch beetle D. simplex exhibits similar behaviour, killing trees stressed by predisposing factors, although recent outbreaks may be cause for rethinking this classification (Werner Reference Werner1986; Langor and Raske Reference Langor and Raske1989a). The mountain pine beetle, D. ponderosae, may remain Canada’s most famous bark beetle (Safranyik and Carroll Reference Safranyik and Carroll2006), given the scope of past and current outbreaks (Campbell et al. Reference Campbell, Alfaro and Hawkes2007).
Each of the sections on spruce beetle, Douglas-fir beetle, and eastern larch beetle are organised around general life histories and factors that affect transitions from endemic to incipient to epidemic levels (Safranyik and Carroll Reference Safranyik and Carroll2006; Weed et al. Reference Weed, Ayres and Bentz2015), as well as notes on management where applicable. The section on mountain pine beetle is considerably shorter, given recent work that captures its natural history, ecology, and population dynamics in depth (e.g., Safranyik and Carroll Reference Safranyik and Carroll2006; Six and Bracewell Reference Six and Bracewell2015). Due to nuances in their individual and population behaviour, regulating factors that may be more important in one species may not be fully addressed in another. A short section on pressing topics for future research in population dynamics concludes each species-specific section. Finally, a concluding section discusses emerging problems for the decades ahead. These topics include aspects of global change such as invasive species, climate change, and ongoing range expansion exhibited by the mountain pine beetle.
The spruce beetle, Dendroctonus rufipennis
Transcontinental within the spruce forests of Canada, the spruce beetle, D. rufipennis Kirby, infests all species of spruce, although white spruce (Picea glauca (Moench) Voss; Pinaceae), Sitka spruce (P. sitchensis (Bongard) Carrière), Engelmann spruce (P. engelmannii Parry ex Engelmann), and Lutz spruce (Picea×lutzii Little) are the most economically important trees. Swaine described several putative beetle species from different species of spruce, but simultaneously postulated that D. borealis, D. obesus, D. engelmanni, and D. piceaperda were one species. Dendroctonus rufipennis may have been omitted from that group because it was erroneously reported in white pine (Pinus strobus Linnaeus; Pinaceae) and jack pines (Pinus banksiana Lambert) (Swaine Reference Swaine1918). Wood (Reference Wood1969) synonymised D. borealis, D. engelmanni, D. obesus, D. piceaperda, and D. similis with D. rufipennis. Adult spruce beetles are ~6 mm long by 3 mm wide, dark brown with rufous or black elytra (Massey and Wygant Reference Massey and Wygant1954; Holsten et al. Reference Holsten, Thier, Munson and Gibson1999).
Spruce beetles typically initiate attacks from late May to early June (Safranyik Reference Safranyik2011). Females are the host selecting sex, and produce seudenol (Vite et al. Reference Vite, Pitman, Fentiman and Kinzer1972), frontalin (Gries et al. Reference Gries, Pierce, Lindgren and Borden1988), verbenene (Gries et al. Reference Gries, Borden, Gries, Lafontaine, Dixon and Wieser1992), and 1-methyl-2-cyclohexen-1-ol (MCOL) (Borden et al. Reference Borden, Gries, Chong, Werner, Holsten and Wieser1996). Frontalin is attractive to both sexes (Furniss et al. Reference Furniss, Baker and Hostetler1976). Spruce beetles also produce an antiaggregation pheromone MCH, which repels attacking beetles (Furniss et al. Reference Furniss, Baker and Hostetler1976). Upon entering the tree, the first 2–3 cm of the egg galleries are constructed on a slight diagonal to the grain (Safranyik et al. Reference Safranyik, Shrimpton and Whitney1983; Safranyik and Linton Reference Safranyik and Linton1999). After mating, females extend the galleries an average length of 13 cm in live trees (Safranyik et al. Reference Safranyik, Shrimpton and Whitney1983). Eggs are laid in groups along the length of both sides of the egg gallery; however, the original 2–3 cm is left free from eggs (Safranyik and Linton Reference Safranyik and Linton1999). On average, eight eggs are laid per cm of gallery in live trees (Massey and Wygant Reference Massey and Wygant1954; Safranyik and Linton Reference Safranyik and Linton1999). In felled trees, an average of 8.5–9.7 eggs is laid per 2.5 cm (Knight Reference Knight1961). Larvae bore horizontally from the egg gallery in groups for the first two instars, after which they form individual mines that can intersect.
The larvae pass through four larval instars. The late fourth instar or pre-pupa is typically the overwintering stage within the host tree, depending on weather conditions (Dyer and Hall Reference Dyer and Hall1977). Once temperatures begin to increase in the spring, larval development continues until late in the summer when pupation occurs. A portion of the adult beetles in standing trees emerge in late August and then travel down the base of the tree to bore in again and overwinter (Massey and Wygant Reference Massey and Wygant1954; Safranyik and Linton Reference Safranyik and Linton1999; British Columbia Ministry of Forests and Range 2012). Knight (Reference Knight1961) estimated that 3–88% of adult beetles emerged from standing trees to bore into the base of the tree for overwintering, although adult beetles in wind-thrown or slash trees tend to overwinter without emerging and repositioning themselves (Dyer and Taylor Reference Dyer and Taylor1971; McCambridge and Knight Reference McCambridge and Knight1972; Schmid and Frye Reference Schmid and Frye1977). Adults exhibit what is thought to be an obligate overwintering reproductive diapause before achieving sexual maturity and attacking new trees (Hansen et al. Reference Hansen, Bentz and Turner2001).
Spruce beetles are associated with a multitude of microorganisms. Leptographium abietinum (Peck) Wingfield (Ophiostomataceae) is the most common fungal associate (Davidson Reference Davidson1955; Ohsawa et al. Reference Ohsawa, Langor, Hiratsuka and Yamaoka2000; Six and Bentz Reference Six and Bentz2003; Aukema et al. Reference Aukema, Werner, Haberkern, Illman, Clayton and Raffa2005). Differences in fungal composition may occur between geographic areas, however, as well as between hosts (i.e., live trees, stumps, deadfall) and population phases (Aukema et al. Reference Aukema, Werner, Haberkern, Illman, Clayton and Raffa2005). Cardoza et al. (Reference Cardoza, Klepzig and Raffa2006) found four major fungi on spruce beetles from the Kenai Peninsula in Alaska, United States of America: L. abietinum; Aspergillus fumigatus Fresenius (Trichocomaceae,); A. nominus Kurtzman, Horn, and Hesseltine; and Trichoderma harzianum Rifai (Hypocreaceae). The latter three species decrease brood survival, although adult beetles exude oral secretions containing bacteria that inhibit the growth of some of the pathogenic species (Cardoza et al. Reference Cardoza, Klepzig and Raffa2006).
Dendroctonus rufipennis primarily attacks downed trees (Safranyik and Linton Reference Safranyik and Linton1999; Safranyik Reference Safranyik2011; British Columbia Ministry of Forests and Range 2012), as live trees present a multitude of defences that a beetle must overcome or avoid. When spruce beetles attack a Sitka or Lutz spruce, for example, the tree produces resin and periderm to exclude the beetles from furthering the attack. If beetles and associated fungi successfully breach the first line of defence, the production of antimicrobial stilbenes and monoterpenes is increased (Werner and Illman Reference Werner and Illman1994). When a tree is stressed (e.g., excessive heat, water excess/deficit, injury, etc.), defensive capacity is reduced. Drought stress, for example, may reduce host tree vigour by affecting water absorption, photosynthesis, and other physiological processes (Kozlowski Reference Kozlowski1982).
Although trees low in vigour may be easier to colonise, there is a trade-off between host quality and host defence with which all species of bark beetles must contend. Stressed trees present lower quality resources for bark beetles. Spruce beetles from poor quality hosts exhibit slower boring rates, slower yolk deposition rates, lower egg production per unit of gallery length, increased egg-free gallery length, and reduced numbers of galleries (Sahota and Thomson Reference Sahota and Thomson1979; Thomson and Sahota Reference Thomson and Sahota1981). Fitness, judged by the reproductive success of an individual, is lower in poor quality spruce beetle populations (Sahota and Ibaraki Reference Sahota and Ibaraki1979). Poor quality beetles are less able to contend with intraspecific and interspecific competition (Sahota and Thomson Reference Sahota and Thomson1979).
Competitive vigour is critical for spruce beetle because competition from other bark beetles is a major limiting factor for endemic populations of D. rufipennis, especially where there is an abundance of downed woody debris (Table 1). Consequences of intraspecific and interspecific competition include reduced reproductive success, reduced beetle vigour (i.e., smaller offspring), and increased mortality (Safranyik and Linton Reference Safranyik and Linton1985; Sahota et al. Reference Sahota, Peet and Ibaraki1987). Ips perturbatus (Eichhoff), for example, is a potential competitor of D. rufipennis that also preferentially attacks recently downed trees or trees affected by stress (e.g., drought or fungi) (Holsten et al. Reference Holsten, Thier, Munson and Gibson1999). Its primary host is Picea Dietrich, but also occurs on Pinus banksiana Lambert and P. contorta (Bright Reference Bright1976). Ips perturbatus is univoltine throughout Alaska (Holsten et al. Reference Holsten, Thier, Munson and Gibson1999) and Alberta, Canada (Robertson Reference Robertson2000). Other important competitors with spruce beetle include Dryocoetes affaber (Mannerheim) in western North America (Gara et al. Reference Gara, Werner, Whitmore and Holsten1995), I. tridens in British Columbia (Poland and Borden Reference Poland and Borden1997), and P. rufipennis in the Great Lakes region (Haberkern and Raffa Reference Haberkern and Raffa2003).
Table 1 Summary of predominant positive and negative feedback processes impacting population dynamics of the spruce beetle, Douglas-fir beetle, eastern larch beetle, and mountain pine beetle.
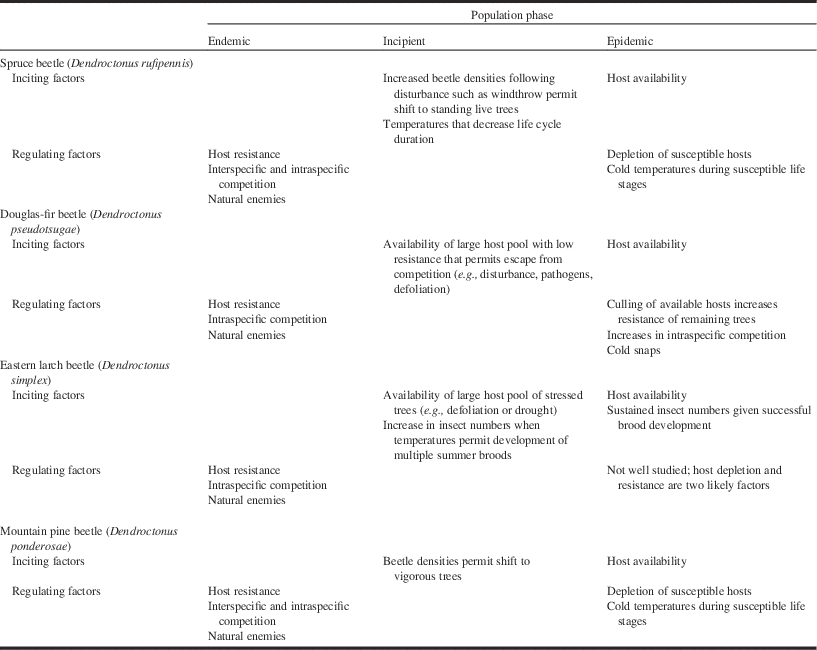
Notes: At the endemic stage, beetles are restricted to unthrifty and moribund trees. At the incipient stage, they reach a threshold by which they begin to colonise predominantly vigourous, live, mature trees. This latter behaviour is characteristic of the epidemic phase. For further detail including references, see text.
In situations where beetle densities exceed the carrying capacity of the woody debris present (Garbutt et al. Reference Garbutt, Hawkes and Allen2006), beetles will begin to attack live trees (Table 1). This behaviour marks the beginning of the incipient-epidemic population phase. Once beetles reach the epidemic phase, host plant defences no longer constrain beetle population growth (Wallin and Raffa Reference Wallin and Raffa2004; Raffa et al. Reference Raffa, Aukema, Bentz, Carroll, Hicke and Turner2008; Boone et al. Reference Boone, Aukema, Bohlmann, Carroll and Raffa2011). Over the past 25 years, for example, the spruce beetle has erupted across vast areas of Alaska, United States of America and the adjacent Yukon Territory of Canada (Berg et al. Reference Berg, Henry, Fastie, Volder and Matsuoka2006; Raffa et al. Reference Raffa, Aukema, Bentz, Carroll, Hicke and Turner2008).
The epidemic phase has been characterised as a minimum of two clumps of five standing infested trees per 5 acres (2 ha) area (Bentz and Munson Reference Bentz and Munson2000). A tree with a diameter at breast height (DBH) of 20 inches (51 cm) may be infested to a height of 35 feet (11 m) (Knight Reference Knight1960). Spruce beetles may also attack stumps below the duff line (Safranyik and Linton Reference Safranyik and Linton1999). Signs of attack may not be seen until the following year or two after attack, when the needles turn greenish-yellow and fall off the tree. On some trees, the only evidence of attack may be red boring dust at the base of the tree instead of pitch tubes (Massey and Wygant Reference Massey and Wygant1954). Spruce beetles often initiate their gallery entrance under a bark flake, making it challenging to see evidence of colonisation.
Spruce beetles attack large-diameter trees during outbreaks, but tree growth rate is a better predictor of beetle success than diameter alone. Trees that were fast growing but had smaller diameters can be more successful at repelling beetle attack versus larger diameter trees that may be growing more slowly (Hard et al. Reference Hard, Werner and Holsten1983). Reductions in radial growth may be a proxy for tree stress, indicating allocation of limited energy reserves to shoot, root, and cone growth at the expense of stemwood production (Waring and Pitman Reference Waring and Pitman1980). Spruce beetles do prefer to attack trees when radial growth is slowed: during warm, dry weather or before the soil thaws in the spring. Heavily attacked trees experience slower radial growth before beetle attack relative to unattacked trees (Hard Reference Hard1987).
The greatest advance in our understanding of spruce beetle population dynamics since the time of Swaine has been the elucidation of some of the mechanisms by which beetle populations transition from endemic to eruptive population phases (Table 1). Temperature can be critical to this population phase transition in which the insects increase in abundance, escaping competitive pressure, and transitioning to successful mass attacks on live trees. Temperature has long been known to mediate development rates. Spruce beetles exhibit a larval development threshold of 6.1 °C and optimal fecundity and development rates at 21.1 °C (Dyer and Hall Reference Dyer and Hall1977; Sahota and Thomson Reference Sahota and Thomson1979). Spruce beetles exhibit a facultative pre-pupal diapause and what is thought to be an obligate adult diapause. When pre-pupae experience threshold cool temperatures of appropriate duration, a facultative diapause arrests development until the following season. If favourably warm environmental temperatures prevail, then spruce beetles proceed to the adult overwintering diapause stage uninterrupted (Hansen et al. Reference Hansen, Bentz and Turner2001, Reference Hansen, Bentz, Powell, Gray and Vandygriff2011). As such, increases in mean annual temperatures and heat accumulation have, in certain areas, allowed the spruce beetle to shift voltinism from the typical two-year or even three-year cycles (Dyer 1969; Massey and Wygant Reference Massey and Wygant1954; Safranyik et al. Reference Safranyik, Shrimpton and Whitney1983; Safranyik and Linton Reference Safranyik and Linton1999; Safranyik Reference Safranyik2011) to one-year life cycles. This shift has resulted in elevated rates of population increase and contributed to recent large-scale outbreaks of the beetle (e.g., Alaska and the western United States of America).
Similar to behaviour exhibited by other aggressive Dendroctonus species such as mountain pine beetle, outbreaks will continue unabated until the host supply is diminished or environmental conditions return the beetle populations to an endemic state (Table 1). In late epidemic phases, for example, non-host trees (e.g., Pinus Linnaeus) may occasionally be attacked, but broods usually do not mature (Massey and Wygant Reference Massey and Wygant1954; McCambridge and Knight Reference McCambridge and Knight1972; Safranyik et al. Reference Safranyik, Shrimpton and Whitney1983). The occurrence of low temperatures before beetles become cold hardy can also cause populations to collapse, although there appears to be both geographic and temporal variation in this trait. Temperatures of only −15 °C and −30 °C have killed most adults and larvae, respectively, in Colorado, United States of America (Massey and Wygant Reference Massey and Wygant1954). Beetles exhibit supercooling points of −12 °C in the summer and −31 °C in the winter in Alaska (Miller et al. Reference Miller, Moser, McGregor, Gregoire, Baiser and Dahlsten1987), although a more recent study in Nova Scotia described supercooling points of −44 °C (Rousseau et al. Reference Rousseau, Bauce, Lavallee and Guertin2012).
Natural enemies may impact spruce beetle populations, although their effect is negligible in outbreak conditions. Spruce beetles have a diverse suite of natural enemies in western North America (Massey and Wygant Reference Massey and Wygant1954; McCambridge and Knight Reference McCambridge and Knight1972; Miller et al. Reference Miller, Moser, McGregor, Gregoire, Baiser and Dahlsten1987; Gara et al. Reference Gara, Werner, Whitmore and Holsten1995). Woodpeckers (Piciformes: Picidae) such as the alpine three-toed woodpecker, Picoides dorsalis dorsalis (Baird); Rocky Mountain hairy woodpecker, Picoides villosus monticola (Anthony); and the downy woodpecker, Picoides pubescens (Linnaeus) have been known to destroy as much as 75% of beetle populations in Colorado (Massey and Wygant Reference Massey and Wygant1954). Thanasimus undatulus Say (Coleoptera: Cleridae) is a primary predator of the spruce beetle in western North America, although it also responds to pheromones of Ips tridens Mannerheim (Poland and Borden Reference Poland and Borden1997). Ips species naturally displace the spruce beetle through competition (McCambridge and Knight Reference McCambridge and Knight1972) and T. undatulus will preferentially feed on I. tridens, therefore potentially reducing the impacts of interspecific competition on the spruce beetle.
Management of eruptive bark beetles such as the spruce beetle thus take one of two forms: (i) indirect or proactive tactics intended to limit the amount of susceptible host material and reduce the probability of rapid population increases; or (ii) direct or reactive tactics intended to destroy infested material and directly reduce beetle populations (Prebble Reference Prebble1955; Carroll et al. Reference Carroll, Shore and Safranyik2006). Indirect tactics in intact stands comprise any activity that will improve tree vigour (e.g., thinning) and thereby increase the resistance of high value, highly susceptible spruce stands (Holsten et al. Reference Holsten, Thier, Munson and Gibson1999). Indirect approaches are also applicable to disturbed stands. During harvesting, keeping stumps low (<45 cm high), and limiting the quantity of large-diameter cull logs and slash will reduce the likelihood of local population increases (Holsten et al. Reference Holsten, Thier, Munson and Gibson1999). For accessible sites with downed trees due to wind or other disturbance, prompt removal of logs (before infestation) can also limit the build-up of populations. Given that most spruce beetle epidemics are linked to extensive windthrow events (Humphreys and Safranyik Reference Humphreys and Safranyik1993; Holsten et al. Reference Holsten, Thier, Munson and Gibson1999) or other widespread forest stressors such as drought (Sherriff et al. Reference Sherriff, Berg and Miller2011), however, the highly intensive nature of indirect tactics can preclude their general application.
Consequently, direct control tactics are often the primary means of limiting spruce beetle impacts. Sanitation harvesting of infested logs and trees is the most common direct control tactic (Humphreys and Safranyik Reference Humphreys and Safranyik1993). Where infestations are accessible to conventional harvesting operations, infested logs/trees can be removed and transported to a mill where prompt processing will cause the mortality of progeny. Infested trees in inaccessible areas can be felled, bucked, piled, and burned to kill broods. Sanitation harvesting can be augmented by tactics that help concentrate beetle populations. Since spruce beetles will readily infest logs, trap trees (i.e., large diameter (>30 cm), felled trees) can be used to attract up to 10× the number of foraging beetles compared to an intact standing tree (Holsten et al. Reference Holsten, Thier, Munson and Gibson1999). Trap trees can be further augmented using semiochemicals. Aggregation pheromones can potentially attract large numbers of beetles. Aggregation pheromones can be deployed alone or in combination with anti-aggregation pheromones to protect high value trees and stands in a “push-pull” manipulation (sensu Borden et al. Reference Borden, Birmingham and Burleigh2006), although there may be regional variation in effective pheromone blends.
The juxtaposition between large outbreaks in the continental east a century ago (Swaine Reference Swaine1918; Prebble Reference Prebble1951) versus Alaska and the Yukon in recent decades (Berg et al. Reference Berg, Henry, Fastie, Volder and Matsuoka2006; Raffa et al. Reference Raffa, Aukema, Bentz, Carroll, Hicke and Turner2008) reminds us that many puzzles await solutions concerning the population dynamics of spruce beetles. Spruce beetles are still present but no longer exhibit landscape-level eruptions in the forests of eastern North America, which may be due to changes in forest structure over the past century. It is difficult to untangle long-term changes in forest composition from confounding changes in climate, however. For example, long-term drought stress from lengthened growing seasons devoid of concomitant increases in precipitation in Alaska has reduced the growth rates and vigour of spruce trees (Barber et al. Reference Barber, Juday and Finney2000), increasing the availability of susceptible hosts (Berg et al. Reference Berg, Henry, Fastie, Volder and Matsuoka2006). El Niño years, for example, coupled with late summer drought are positively correlated with the occurrence of spruce beetle outbreaks (Sherriff et al. 2011). Yet climatic changes that potentially yield above-average levels of precipitation could also alter this system. An increase in precipitation as snow in the northern parts of the range of the spruce beetle, for example, predicted under some climate change scenarios (e.g., Intergovernmental Panel on Climate Change 2014), may favour beetle survival because snow can insulate overwintering beetles from lethal minimum temperatures and minimise woodpecker predation (McCambridge and Knight Reference McCambridge and Knight1972). Similarly, increased snow loading combined with warmer spring temperatures may allow beetles to attack trees before their roots have thawed, thereby improving attack success. The net effect of warmer temperatures and altered precipitation regimes on the spruce beetle may be a greater likelihood of population increases and landscape-scale outbreaks.
The Douglas-fir beetle, Dendroctonus pseudotsugae
The Douglas-fir beetle preferentially colonises downed and low-vigour Douglas-firs (Pseudotsuga menziesii (Mirbel) Franco; Pinaceae) under endemic conditions (Hopkins Reference Hopkins1909b; Lejeune et al. Reference Lejeune, McMullen and Atkins1961; Furniss and Carolin Reference Furniss and Carolin1977; Fredricks and Jenkins Reference Fredricks and Jenkins1988; Schmitz and Gibson Reference Schmitz and Gibson1996; Powers et al. Reference Powers, Sollins, Harmon and Jones1999; Humphreys Reference Humphreys2000). It is considered the primary insect enemy of Douglas-fir (Furniss and Carolin Reference Furniss and Carolin1977). The beetle will also attack, however, standing western larch (Larix occidentalis Nuttall; Pinaceae). Logs of western hemlock (Tsuga heterophylla (Rafinesque) Sargent; Pinaceae), western redcedar (Thuja plicata Donn ex Don; Pinaceae), and Brewer spruce (Picea breweriana Watson; Pinaceae) are occasionally colonised. Douglas-fir, however, represents the only potential host species that can be successfully colonised by the beetle when alive (Furniss Reference Furniss1976).
Mature Douglas-fir beetles emerge, disperse and colonise new host material in early spring. Mated females may re-emerge during the early summer and establish a second “sibling” or “sister” brood in additional host material. Sister broods are established by the same parent beetles as the first brood, but are sometimes referred to as a second or partial “generation” (Hopkins Reference Hopkins1909b; Wood Reference Wood1982; Lessard and Schmid Reference Lessard and Schmid1990; Schmitz and Gibson Reference Schmitz and Gibson1996). Approximately 20% of female beetles are reported to re-emerge in order to lay a second brood (Schmitz and Gibson Reference Schmitz and Gibson1996). Callow adults of the first brood are normally present in host trees by early August but remain under the bark to sclerotise and overwinter. Sister broods overwinter as late instars and complete development the following spring. By early summer, the sister brood completes development, emerges, and colonises host material. This timing of emergence corresponds with the re-emergence of spring-attacking females and thus, a second set of host material may be colonised cooperatively by re-emergent and newly emergent adult beetles (Hopkins Reference Hopkins1909b; Furniss Reference Furniss1976; Wood Reference Wood1982; Humphreys Reference Humphreys2000).
Similar to the spruce beetle, the Douglas-fir beetle thrives in physiologically compromised host material with reduced capacities for an oleoresin defensive response (Rudinsky Reference Rudinsky1966) and diminished carbohydrate reserves for monoterpene production (Webb and Karchesy Reference Webb and Karchesy1977). At endemic levels, the beetle prefers to infest the stumps, fallen branches, wind-thrown stems, and logging slash of Douglas-fir as well as low-vigour standing trees that have been severely injured by wind or snow breakage, fire, lightening, root disease, drought, and defoliation (Table 1; Hopkins Reference Hopkins1909b; Lejeune et al. Reference Lejeune, McMullen and Atkins1961; Furniss and Carolin Reference Furniss and Carolin1977; Fredricks and Jenkins Reference Fredricks and Jenkins1988; Schmitz and Gibson Reference Schmitz and Gibson1996; Powers et al. Reference Powers, Sollins, Harmon and Jones1999; Humphreys Reference Humphreys2000). Indeed, the relationship between Douglas-fir infected with various species of root rot fungi (e.g., Armillaria solidipes Peck (Physalacriaceae) and Phellinus weirii (Murrill) Gilbertson (Hymenochaetaceae)) and attack by Douglas-fir beetle is most pronounced during endemic beetle population phases (Furniss et al. Reference Furniss, McGregor, Foiles and Partridge1979, Reference Furniss, Livingston and McGregor1981; Humphreys Reference Humphreys2000). A similar relationship exists for Douglas-firs that have experienced heavy defoliation by western spruce budworm Choristoneura occidentalis (Freeman) (Lepidoptera: Tortricidae) or the Douglas-fir tussock moth Orgyia pseudotsugae (McDunnough) (Lepidoptera: Lymantriidae) (Wright et al. Reference Wright, Berryman and Wickman1984; Fredricks and Jenkins Reference Fredricks and Jenkins1988).
Reduced host vigour allows Douglas-fir beetles to colonise Douglas-fir material at low attack densities of 43–86 attacks/m2 (4–8 attacks/ft2) and reduce intraspecific competition, increasing reproductive success (Atkins and McMullen Reference Atkins and McMullen1960; McMullen and Atkins Reference McMullen and Atkins1961; Schmitz and Rudinsky Reference Schmitz and Rudinsky1968; Furniss et al. Reference Furniss, Livingston and McGregor1981; Wright et al. Reference Wright, Berryman and Wickman1984; Powers et al. Reference Powers, Sollins, Harmon and Jones1999). The availability of stressed host material on the landscape is often severely limited, however. Thus, material is often colonised at densities sufficient to promote intraspecific competition and limit beetle reproductive success, constraining population growth (Furniss et al. Reference Furniss, McGregor, Foiles and Partridge1979, Reference Furniss, Livingston and McGregor1981). Standing, vigorous trees located near to the attractant source of stressed material are also often attacked, but successful beetle colonisation often fails due to the intact oleoresin defences of the healthy trees. Such trees therefore act as beetle “sinks” and further serve to limit beetle reproductive success and maintain low beetle densities (Johnson and Belluschi Reference Johnson and Belluschi1969; Furniss et al. Reference Furniss, McGregor, Foiles and Partridge1979).
The shift of Douglas-fir beetle populations from endemic to incipient-epidemic phases occurs when an abundance of low-vigour host material fosters conditions for beetle success; that is, large numbers of beetles can colonise at low densities across a broad resource with reduced intraspecific competition (Table 1). The influx of such host material may occur from acute disturbances such as low intensity fires, logging practices creating large amounts of slash, or wind-throw events (Atkins and McMullen Reference Atkins and McMullen1960; Furniss and Carolin Reference Furniss and Carolin1977; Powers et al. Reference Powers, Sollins, Harmon and Jones1999). The importance of wind-throw for creating suitable breeding material is more prevalent in the coastal and northern Rocky Mountain Douglas-fir forests where trees tend to be larger and more prone to wind-throw relative to the southern Rocky Mountains where trees are smaller and more wind firm (Furniss et al. Reference Furniss, Livingston and McGregor1981). Even small areas or “micro-sites” within a larger stand, however, can catalyse beetle activity that will spread to the larger stand (Negrón et al. Reference Negrón, Anhold and Munson2001).
In addition to acute inputs of host material, chronic declines in stand vigour may create favourable conditions for increases in beetle abundance. Older stands of Douglas-fir with declining vigour are also associated with elevated levels of beetle attack, for example (Furniss et al. Reference Furniss, Livingston and McGregor1981). High levels of activity may also be noted in stands with a basal areas of Douglas-fir (Furniss et al. Reference Furniss, McGregor, Foiles and Partridge1979; Negrón Reference Negrón1998; Negrón et al. Reference Negrón, Schaupp, Gibson, Anhold, Hansen and Thier1999; Powers et al. Reference Powers, Sollins, Harmon and Jones1999) greater than surrounding areas (Furniss et al. Reference Furniss, McGregor, Foiles and Partridge1979, Reference Furniss, Livingston and McGregor1981; Hadley and Veblen Reference Hadley and Veblen1993; Negrón Reference Negrón1998). Dense stands may be of lower vigour (Negrón Reference Negrón1998) due to moisture stress (Furniss et al. Reference Furniss, Livingston and McGregor1981). Additionally, dense stands provide increased shading that allows the beetles to colonise the entire tree bole because Douglas-fir beetles tend to avoid sunlit aspects of standing or downed host material (Furniss et al. Reference Furniss, Livingston and McGregor1981) that desiccate their tunnels (Swaine Reference Swaine1918). At a stand level, trees on southern aspects experience greater seasonal and daily declines in oleoresin exudation pressure than trees growing on northern aspects. Trees growing at lower elevations and on east, south, or southwest aspects also tend to be drier (Powers et al. Reference Powers, Sollins, Harmon and Jones1999) and are more often associated with increased beetle activity (Schmitz and Gibson Reference Schmitz and Gibson1996; Powers et al. Reference Powers, Sollins, Harmon and Jones1999; Humphreys Reference Humphreys2000).
Populations of Douglas-fir beetles in low-vigour material may increase rapidly (Atkins and McMullen Reference Atkins and McMullen1960; McMullen and Atkins Reference McMullen and Atkins1961; Schmitz and Rudinsky Reference Schmitz and Rudinsky1968; Furniss et al. Reference Furniss, Livingston and McGregor1981; Wright et al. Reference Wright, Berryman and Wickman1984; Powers et al. Reference Powers, Sollins, Harmon and Jones1999); even >10-fold over a single generation (Atkins and McMullen Reference Atkins and McMullen1960). In fact, outbreaks can be initiated after just two generations of beetles reproducing in large volumes of wind-thrown timber (Wright et al. Reference Wright, Berryman and Wickman1984). Increasing Douglas-fir beetle populations rapidly exploit all available vigour-impaired host material leading to an increased frequency of attacks on standing trees that may be of reduced health and vigour but still possess intact resin defence systems (Johnson and Belluschi Reference Johnson and Belluschi1969; Furniss et al. Reference Furniss, Livingston and McGregor1981; Humphreys Reference Humphreys2000). However, defences of weakened trees are quickly overwhelmed by the sheer number of attacking beetles, with the result being the death of some proportion of the standing timber (Johnson and Belluschi Reference Johnson and Belluschi1969; Furniss et al. Reference Furniss, McGregor, Foiles and Partridge1979; Powers et al. Reference Powers, Sollins, Harmon and Jones1999). The most vigorous trees have a greater threshold for repelling beetle attacks before tree mortality occurs (Furniss et al. Reference Furniss, McGregor, Foiles and Partridge1979).
Douglas-fir trees that tend to be killed early during the incipient-epidemic population phase of Douglas-fir beetle outbreaks are generally large (20+ cm diameter at breast height), mature (120+ years old), exhibit thick bark and phloem, demonstrate low growth rates during the previous 5–10 years, and have compromised oleoresin defence capabilities (Rudinsky Reference Rudinsky1966; Furniss et al. Reference Furniss, McGregor, Foiles and Partridge1979, Reference Furniss, Livingston and McGregor1981; Lessard and Schmid Reference Lessard and Schmid1990; Powers et al. Reference Powers, Sollins, Harmon and Jones1999; Shore et al. Reference Shore, Safranyik and Riel1999; Humphreys Reference Humphreys2000; Garrison-Johnston et al. Reference Garrison-Johnston, Moore, Cook and Niehoff2003). Brood production in larger diameter trees is enhanced compared to low diameter trees, however, and supports the transition of the beetle population to the epidemic phase (Humphreys Reference Humphreys2000).
During the epidemic population phase, larger areas of standing timber are successfully attacked, colonised, and killed by the Douglas-fir beetle (Table 1). The large numbers of beetles present within a stand are generally able to attack and overcome the defences of even the most vigorous trees. Accordingly, no relationship exists between tree vigour and the ability to survive beetle attacks during the epidemic phase (Powers et al. Reference Powers, Sollins, Harmon and Jones1999; Garrison-Johnston et al. Reference Garrison-Johnston, Moore, Cook and Niehoff2003). Hence, larger areas of the surrounding stand or forest are affected by beetle attack and Douglas-fir mortality. Mortality across thousands of hectares of Douglas-fir forest is not uncommon during outbreaks (Schmitz and Gibson Reference Schmitz and Gibson1996). It is estimated that on an annual basis, the Douglas-fir beetle is responsible for killing hundreds of millions of board feet of timber throughout the range of Douglas-fir (Furniss and Carolin Reference Furniss and Carolin1977; Wood Reference Wood1982). Outbreaks of the Douglas-fir beetle in standing timber typically last from two to four years, but may last longer in some instances (Furniss et al. Reference Furniss, McGregor, Foiles and Partridge1979; Schmitz and Gibson Reference Schmitz and Gibson1996).
The decline of Douglas-fir beetle populations from epidemic back to endemic levels is tightly linked to a coincident density-dependent decline in suitable host material (i.e., a relative increase in stand resistance), increases in intraspecific competition among Douglas-fir beetle larvae within successfully killed hosts, and subsequent declines in beetle reproductive success and offspring production (Table 1; Atkins and McMullen Reference Atkins and McMullen1960; McMullen and Atkins Reference McMullen and Atkins1961; Johnson and Belluschi Reference Johnson and Belluschi1969; Furniss et al. Reference Furniss, McGregor, Foiles and Partridge1979; Wright et al. Reference Wright, Berryman and Wickman1984; Powers et al. Reference Powers, Sollins, Harmon and Jones1999; Garrison-Johnston et al. Reference Garrison-Johnston, Moore, Cook and Niehoff2003). With each successive year of beetle attack, the most susceptible trees are removed from the population such that the most vulnerable trees in a stand are usually depleted within three years (Garrison-Johnston et al. Reference Garrison-Johnston, Moore, Cook and Niehoff2003). This cull results in a significant increase in the relative resistance to beetle attack among the remaining trees with each year of tree mortality (Furniss et al. Reference Furniss, McGregor, Foiles and Partridge1979). The increased level of resistance among remaining trees translates into higher thresholds of beetle attack required to overcome host defences (Johnson and Belluschi Reference Johnson and Belluschi1969). The increased beetle attack densities needed to colonise such trees creates increased intraspecific competition among developing larvae that is detrimental to brood production (McMullen and Atkins Reference McMullen and Atkins1961; Schmitz and Rudinsky Reference Schmitz and Rudinsky1968; Powers et al. Reference Powers, Sollins, Harmon and Jones1999). As a result of such competition (i.e., 161–183/m2 (15–17 attacks/ft2); Atkins and McMullen Reference Atkins and McMullen1960), many vigorous trees killed by attacking beetles serve as beetle sinks rather than beetle sources, slowing population growth (Johnson and Belluschi Reference Johnson and Belluschi1969; Furniss et al. Reference Furniss, McGregor, Foiles and Partridge1979, Reference Furniss, Livingston and McGregor1981). Under such conditions, Furniss et al. (Reference Furniss, McGregor, Foiles and Partridge1979) found that 55% of standing Douglas-fir trees that were attacked and killed by Douglas-fir beetles exhibited brood production that was either below or at parity with the number of attacking beetles needed to kill the tree.
Natural enemies known to prey on Douglas-fir beetles include Enoclerus sphegeus (Fabricius) (Coleoptera: Cleridae), Thanasimus undatulus (Say) (Coleoptera: Cleridae), Temnoscheila chlorodia (Mannerheim) (Coleoptera: Trogossitidae), Coeloides brunneri Viereck (Hymenoptera: Braconidae), and Medetera aldrichii Wheeler (Diptera: Dolichopodidae) (Furniss and Carolin Reference Furniss and Carolin1977). None of these insects have been found to be important regulatory agents during Douglas-fir beetle outbreaks, however (Furniss et al. Reference Furniss, McGregor, Foiles and Partridge1979). As with other Dendroctonus species, natural enemies may only impact populations of Douglas-fir beetles at small scales when beetle populations are at low densities (Powers et al. Reference Powers, Sollins, Harmon and Jones1999).
Efforts to manage the Douglas-fir beetle typically focus on indirect tactics to limit the rapid increase of populations. Experimental thinning of overly dense Douglas-fir stands has been shown to substantially reduce Douglas-fir beetle infestation by increasing tree vigour, reducing stem shading, and limiting the availability of low-vigour host material such as wind-throw, breakage, and suppressed trees (Williamson and Price Reference Williamson and Price1971). It is important, however, that thinning operations avoid collateral damage to remaining standing trees. The amount of slash left within the stand must also be carefully managed following harvesting operations (Swaine Reference Swaine1918). The reported preference of Douglas-fir beetles for logging slash is such that beetles avoid attacking standing timber so long as a sufficient supply of slash is available. Thus, it has been recommended to balance the availability of logging slash so that enough is available to prevent beetles from attacking standing timber while disallowing beetle populations to increase dramatically (Lejeune et al. Reference Lejeune, McMullen and Atkins1961). Similar to the spruce beetle, sanitation harvesting together with the use of trap trees and semiochemical manipulation are common direct control tactics applied to reduce Douglas-fir beetle populations (Humphreys Reference Humphreys2000; Laidlaw et al. Reference Laidlaw, Prenzel, Reid, Fabris and Wieser2003; Ross and Wallin Reference Ross and Wallin2008; Gillette et al. Reference Gillette, Mehmel, Webster, Mori, Erbilgin and Wood2009).
As with spruce beetle, variations in weather and climate can impact changes in abundance in space and time for Douglas-fir beetles. Swaine’s qualitative association of moderately warm weather with increased populations of bark beetles would seem to hold true for Douglas-fir beetle. Early, warm spring weather, for example, may stimulate the beetles to emerge quickly and synchronously. However, if cool, wet weather sets in shortly thereafter, the emergent beetles are left unable to attack host trees. Exposed to the elements, beetles may be unable to tunnel into host bark for protection and thus succumb to predation and exposure (Atkins and McMullen Reference Atkins and McMullen1960). Summer temperatures, which dictate the rate of larval development, can reduce the potential for the development of population outbreaks if too cool (Johnson and Belluschi Reference Johnson and Belluschi1969). During the fall, sudden cold snaps can cause 60–80% mortality to the overwintering beetle population (Atkins and McMullen Reference Atkins and McMullen1960). Such temperatures can exert different rates of mortality to the different overwintering life-stages (Johnson and Belluschi Reference Johnson and Belluschi1969).
There are several aspects of the lifecycle and reproductive biology of the Douglas-fir beetle that have not been well studied to date, despite the ease with which it can be captured in traps for experimental purposes. The beetle is reported to exhibit a univoltine life cycle (Wood Reference Wood1982; Schmitz and Gibson Reference Schmitz and Gibson1996; Humphreys Reference Humphreys2000). This life cycle is putatively enforced by an obligate reproductive diapause of newly developed brood adults (Furniss Reference Furniss1976). This obligatory diapause period has never been empirically characterised, however. The habits of summer-emergent brood adults that overwintered as larvae to engage in host attack without an overwintering period as adults (Hopkins Reference Hopkins1909b; Furniss Reference Furniss1976; Schmitz and Gibson Reference Schmitz and Gibson1996; Humphreys Reference Humphreys2000) suggests that diapause in this beetle cannot be obligate. Similarly, the impacts of partial or complete sister broods on the population dynamics of this insect are unknown, as is the case for many bark beetles (McMullen and Atkins Reference McMullen and Atkins1961; Anderbrant Reference Anderbrant1989; Dworschak et al. Reference Dworschak, Gruppe and Schopf2014; Öhrn et al. Reference Öhrn, Långström, Lindelöw and Björklund2014). The establishment of second sibling broods by parent beetles has the potential to ameliorate the detrimental effects of intraspecific competition among larvae of the first brood, and could have important ramifications for adult reproductive success and population dynamics (McMullen and Atkins Reference McMullen and Atkins1961). Microorganisms also mediate reproductive success, although relatively few studies have attempted to characterise microorganisms associated with the Douglas-fir beetle (Lu et al. Reference Lu, Allen and Bollen1957; Lewinsohn et al. Reference Lewinsohn, Lewinsohn, Bertagnolli and Patridge1994; Solheim and Krokene Reference Solheim and Krokene1998). Finally, research that investigates how the Douglas-fir beetle may react to future climate scenarios would be beneficial. In particular, efforts to understand the effect that changing climatic patterns may have on the vitality of Douglas-fir forests could provide useful insights into the potential tree-killing behaviour of the Douglas-fir beetle in the forthcoming decades.
The eastern larch beetle, Dendroctonus simplex
The eastern larch beetle has traditionally been considered a nonaggressive bark beetle that colonises eastern larch (tamarack) (Larix laricina (Du Roi) Koch; Pinaceae) (Hopkins Reference Hopkins1909b). Eastern larch beetles typically demonstrate a propensity for attacking tamaracks that are weakened, injured, dying, or recently dead (Hopkins Reference Hopkins1909b; Wood Reference Wood1982). Commonly, tamaracks colonised by eastern larch beetles are stressed by a local disturbance event such as wind throw, ice damage, flooding, timber harvest, or old age (Langor and Raske Reference Langor and Raske1988a, Reference Langor and Raske1989a, Reference Langor and Raske1989b). In recent decades, however, the eastern larch beetle has undergone landscape-level outbreaks and has demonstrated that the beetle should potentially be reconsidered as an aggressive species (Werner Reference Werner1986; Langor and Raske Reference Langor and Raske1989a; McKee Reference McKee2015). Due to a historic designation as a bark beetle of low economic importance, studies on the natural history and ecology of eastern larch beetles have been extremely limited (Seybold et al. Reference Seybold, Albers and Katovich2002). As such, knowledge of the mechanisms that allow the eastern larch beetle to transition from endemic to epidemic population phases are not well understood. However, historical reports of eastern larch beetle natural history during localised beetle activity as well as more recent research undertaken during landscape-level outbreaks provides some insight to the potential mechanisms that govern the population dynamics of this insect (McKee Reference McKee2015).
Adult eastern larch beetles generally begin to emerge from natal hosts in mid-May. Emergence generally peaks rapidly, but continues into the first week of June. During very warm springs, emergence may begin the first week of May and be complete by the end of May. Beetle attack on new host trees or downed material occurs within a few days of beetle emergence; however peak attack periods do not always coincide with peak emergence (McKee Reference McKee2015). Eggs of the first brood are laid within a few days of host material being attacked. Similar to Douglas-fir beetles, adult females will often re-emerge from fully colonised host material to establish a second, and, occasionally, a third sibling brood. Larvae develop quickly, such that adult progeny of the first sibling brood are often present within host material by early July. The presence of adult progeny of the second brood is variable and depends on the year and the timing of brood establishment by the re-emergent adult beetles. Accordingly, adult progeny can first be found in host material ranging from mid-July to mid-September. Development of the third sibling brood to the adult stage is also quite variable and can be as early as late-August of the year of oviposition or as late as early-summer of the year following oviposition (McKee Reference McKee2015).
Similar to D. rufipennis, brood adults of the eastern larch beetle may reposition themselves within standing natal host trees before winter by emerging from the pupal chamber, descending to the base of the tree, and boring back under the bark to create hibernal galleries in which to overwinter. The pre-winter emergence of adult progeny of the first sibling brood typically begins one to two weeks after development to the brood adult life-stage occurs, but may be up to four weeks later for the brood adults of the second and third sibling broods. In some cases, brood adults of sibling broods do not engage in pre-winter emergence (Hopkins Reference Hopkins1909b; Swaine Reference Swaine1911; Simpson Reference Simpson1929; Prebble Reference Prebble1933; Werner Reference Werner1986; Langor and Raske Reference Langor and Raske1987a, Reference Langor and Raske1987b).
Similar to other bark beetles, and consistent with Swaine’s models of bark beetle abundance, the abundance of eastern larch beetles appears to be highly correlated with preferred resource availability (Table 1). Localised disturbance events (e.g., snow breakage, wind-throw) injure single or small groups of tamaracks and create a preferred resource for endemic eastern larch beetles that is limited in quantity, ephemeral, and is spatially separated across the landscape (Hopkins Reference Hopkins1909b; Langor and Raske Reference Langor and Raske1988a, Reference Langor and Raske1989a, Reference Langor and Raske1989b). The use of small pockets of stressed tamaracks apparently sustains endemic eastern larch beetle populations and temporarily facilitates a short period of increased beetle activity lasting one to three years. Such increases in abundance remain largely confined to the stressed tamarack resource, however (Langor and Raske Reference Langor and Raske1988a). Localised, short-term beetle activity is considered typical for eastern larch beetles and has been reported for over 100 years (Hopkins Reference Hopkins1909b). In general, eastern larch beetle populations decline to unnoticeable levels when the supply of stressed tamaracks (or material) has been fully used and the only available resources are standing, healthy trees. The oleoresin defence system of living tamaracks, as well as of recently dead host material, is known to cause mortality among attacking adult beetles as well as developing larvae, but has not been studied in detail (Simpson Reference Simpson1929; Langor and Raske Reference Langor and Raske1988b; Werner Reference Werner1995).
Disturbance events that act on a broad rather than local scale may compromise the health of tamaracks across larger areas and provide increased amounts of material that are suitable for eastern larch beetle colonisation and reproduction. Widespread defoliation of tamaracks appears to be important for inciting landscape-scale outbreaks of eastern larch beetles, for example (Werner Reference Werner1986; Langor and Raske Reference Langor and Raske1988a, Reference Langor and Raske1989b), as radial growth of tamarack is significantly reduced by repeated defoliation events (Benoit and Blais Reference Benoit and Blais1988; Tailleux and Cloutier Reference Tailleux and Cloutier1993). The increase in reproductive output within stressed host trees allows beetle populations to surpass critical population density thresholds. Breaching these thresholds permit beetles to attack, colonise, and successfully reproduce within large expanses of healthy, vigorous larch forests (Langor and Raske Reference Langor and Raske1989a, Reference Langor and Raske1989b).
In the mid-1970s and early-1980s eastern larch beetles exhibited the first known landscape-level epidemics. These outbreaks, lasting five to six years each (Werner Reference Werner1986; Langor and Raske Reference Langor and Raske1988a), affected more than 3.3 million ha of tamarack forest in Alaska and killed more than 1.4 million m3 of tamarack in the Canadian Maritimes. While the extent of beetle activity and tamarack mortality was more than what Swaine would have predicted, the patterns followed predisposing actions of defoliators written about in his text (Swaine Reference Swaine1918). These eastern larch beetle epidemics followed episodes of repeated and wide-spread defoliation of tamaracks by spruce budworm (Choristoneura fumiferana (Clemens); Lepidoptera: Tortricidae), larch sawfly (Pristiphora erichsonii (Hartig); Hymenoptera: Tenthredinidae), larch budmoth (Zeiraphera Treitschke; Lepidoptera: Tortricidae), or larch casebearer (Coleophora laricella (Hübner); Lepidoptera: Coleophoridae) (Werner Reference Werner1986; Langor and Raske Reference Langor and Raske1989a, Reference Langor and Raske1989b).
When attacking healthy tamaracks, eastern larch beetles will target a variety of tree sizes (Seybold et al. Reference Seybold, Albers and Katovich2002) but demonstrate an initial preference for the largest trees (Hopkins Reference Hopkins1909b; Werner Reference Werner1986; Langor and Raske Reference Langor and Raske1989a, Reference Langor and Raske1989b; McKee Reference McKee2015). In other bark beetles, the largest trees are shown to increase the reproductive success of parent beetles and facilitate the shifts between population phases (e.g., incipient-epidemic to epidemic; Table 1; Amman Reference Amman1972; Amman and Pace Reference Amman and Pace1976; Humphreys Reference Humphreys2000). Eastern larch beetles also exhibit improved reproductive success in larger diameter tamaracks (Werner Reference Werner1986). Specific studies on host–insect interactions between eastern larch beetles and tamaracks that determine optimum reproductive success as a factor of host size are lacking, however. Defensive resinosis responses in colonised tamaracks exert an antixenotic effect on eastern larch beetle offspring (Werner Reference Werner1986; Langor and Raske Reference Langor and Raske1988b), but relationships between tree age, size, vigour, and beetle reproductive success are not yet fully elucidated (McKee Reference McKee2015).
Similarly, while the predisposition of eastern larch beetle activity by defoliating insects has been well noted, the interactions between the eastern larch beetle and potential competitors have received limited study. Eastern larch beetles are often noted as the dominant or only species of bark beetle found within the main stem of colonised tamaracks during endemic and epidemic population phases (Dodge Reference Dodge1938; Langor Reference Langor1991; Seybold et al. Reference Seybold, Albers and Katovich2002). Relatively few species of bark beetles colonise tamarack, so potential competitors are limited to phloem-feeding species such as Polygraphus rufipennis (Kirby), Scolytus piceae (Swaine), Crypturgus pusillus (Gyllenhal), and Orthotomicus caelatus (Eichhoff) (Dodge Reference Dodge1938; Rose and Lindquist Reference Rose and Lindquist1980; Wood Reference Wood1982; Langor Reference Langor1991). These species are all polyphagous, however, and are not exclusive colonisers of tamarack (Wood Reference Wood1982). Resource partitioning may also minimise competitive interactions when larger species (e.g., eastern larch beetle) colonise the thicker phloem of the main stem while smaller species (e.g., Polygraphus rufipennis) take advantage of the thinner phloem in the upper stem and larger branches.
Interactions between the eastern larch beetle and associated natural enemies and microorganisms are likewise only documented in a limited number of studies (Langor and Raske Reference Langor and Raske1988b; Langor Reference Langor1991). In general, it appears that overall mortality inflicted on developing eastern larch beetle larvae by parasitoid species such as Roptrocerus xylophagorum (Ratzburg) (Hymenoptera: Pteromalidae) can be significant (i.e., up to 37%), although these are not expected to suppress outbreaking populations. Fungal pathogens can also be responsible for up to 15% larval mortality (Langor and Raske Reference Langor and Raske1988b), although the breadth and types of microorganisms associated with eastern larch beetles have not been well characterised (Jacobs et al. Reference Jacobs, Wingfield and Bergdahl1997). Additional studies on natural causes of eastern larch beetle mortality and how microbes may mediate insect-host dynamics are sorely needed.
Due to the limited number of landscape-scale outbreaks of eastern larch beetles, the mechanisms that precipitate the decline in beetle populations from epidemic back to endemic phases have not been determined (Table 1). One potential mechanism that may act to reduce the reproductive success of eastern larch beetles during epidemics includes the gradual increase in the level of host resistance remaining among living trees as less vigorous trees are culled from the forest, similar to the phenomenon exhibited in outbreaks of Douglas-fir beetle. It is possible that the resin-induced toxicity of vigorous trees may act to curtail beetle reproductive success such that proportionately more tamaracks act as beetle sinks than beetle sources and eventually cause the collapse of beetle populations. Additionally, below-average winter temperatures may cause increased mortality to overwintering adult and sub-adult life-stages. Eastern larch beetles are extremely cold-hardy, however, with supercooling points of −49 °C and −42 °C for larvae and adults, respectively, in December (Venette and Walter Reference Venette and Walter2008).
Because tamarack has not historically been managed for timber, there is a paucity of studies that have evaluated management tactics against eastern larch beetle (Seybold et al. Reference Seybold, Albers and Katovich2002). Swaine’s recommendations of timely sanitation of logging slash or storm-damaged material would appear to be sensible practices due to the beetle’s preference for breeding in downed material, and could even be extended to the use of trap trees (Swaine Reference Swaine1918). No studies have evaluated silvicultural practices such as thinning in tamarack stands, although care would be needed to avoid injury to the shallow roots of tamaracks during such operations (Seybold et al. Reference Seybold, Albers and Katovich2002). Pheromone and host-volatile blends can trap large numbers of beetles and could be incorporated into a programme of integrated pest management, although the characterisation of the pheromone profile of eastern larch beetle is still not complete (Baker et al. Reference Baker, Hostetler and Furniss1977; Barkawi et al. Reference Barkawi, Franke and Blomquist2003).
Clearly, more research is required to fully understand the population dynamics of the eastern larch beetle (McKee Reference McKee2015). The mechanisms by which it is able to maintain viable populations during the endemic phase (e.g., competition versus facilitation via other bark beetle species), and how beetles in the endemic phase interact with the resin defence system of tamarack, is not yet known. Similar to Douglas-fir beetle, the effects of sibling broods on the overall population dynamics of this insect are also not well understood (McKee Reference McKee2015). Some studies report high levels of late-fall, cold-induced mortality to the sub-adult life-stages of the second sibling brood (Langor and Raske Reference Langor and Raske1987b) that result in this brood contributing less than 1% to the reproductive adult beetle population (Langor Reference Langor1987). In contrast, there is evidence to suggest that sibling broods can successfully overwinter (Simpson Reference Simpson1929; McKee Reference McKee2015). Recent laboratory studies indicate that the reproductive potential of spring-emergent adult beetles from different sibling broods is equivalent under laboratory conditions, suggesting that altered voltinism in a changing climate could exacerbate outbreaks of this insect (F.R.M. and B.H.A., personal observation).
The potential for shifts in voltinism is currently being explored for the eastern larch beetle (McKee and Aukema Reference McKee and Aukema2015a, Reference McKee and Aukema2015b). Swaine thought most Dendroctonus species to be univoltine in Canada, without mention of physiological enforcing mechanisms. Reproductive diapause in the eastern larch beetle was previously thought to be obligate such that brood adults had to overwinter before becoming reproductively viable (Langor and Raske Reference Langor and Raske1987a). As such, eastern larch beetles have been assumed to possess a univoltine lifecycle with only a single, spring-emergent reproductive generation per year (Hopkins Reference Hopkins1909b, Langor and Raske Reference Langor and Raske1987a). Recent evidence indicates that some insects within a population may lack an obligate reproductive diapause, as some brood adults can become reproductively mature without an overwintering period (McKee and Aukema Reference McKee and Aukema2015b). This trait may allow some insects to reproduce in a bivoltine or semivoltine manner given appropriate environmental conditions, although the possibility and frequency of this phenomenon under natural field conditions is still being evaluated (McKee Reference McKee2015).
The mountain pine beetle, Dendroctonus ponderosae
Currently, the most significant bark beetle of concern to North American forests is the mountain pine beetle. The behaviour and brood productivity of mountain pine beetle at endemic levels is still not well understood (Bleiker et al. Reference Bleiker, O'Brien, Smith and Carroll2014), although it appears that endemic populations can be facilitated by species that are otherwise competitors at higher population densities (Table 1; Rankin and Borden Reference Rankin and Borden1991; Smith et al. Reference Smith, Carroll and Lindgren2011). Due to the extent and impact of mountain pine beetle at the landscape-scale, studies on factors influencing outbreak dynamics, such as changes in temperature or precipitation regimes, are much more abundant (Aukema et al. Reference Aukema, Carroll, Zheng, Zhu, Raffa and Moore2008; Chapman et al. Reference Chapman, Veblen and Schoennagel2012; Preisler et al. Reference Preisler, Hicke, Ager and Hayes2012; Sambaraju et al. Reference Sambaraju, Carroll, Zhu, Stahl, Moore and Aukema2012; Creeden et al. Reference Creeden, Hicke and Buotte2014).
One hundred years ago, mountain pine beetle was responsible for “several” large outbreaks in British Columbia’s pine forests, and several more outbreaks have been recorded in the past century (Swaine Reference Swaine1918; Hopping and Mathers Reference Hopping and Mathers1945; Aukema et al. Reference Aukema, Carroll, Zhu, Raffa, Sickley and Taylor2006; Campbell et al. Reference Campbell, Alfaro and Hawkes2007; Hrinkevich and Lewis Reference Hrinkevich and Lewis2011). The most recent outbreak in British Columbia and Alberta is the largest in recorded history (Raffa et al. Reference Raffa, Aukema, Bentz, Carroll, Hicke and Turner2008), converting affected forests from carbon sinks to carbon sources (Kurz et al. Reference Kurz, Dymond, Stinson, Rampley, Neilson and Carroll2008) and even impacting regional weather through concomitant reduced evapotranspiration (Maness et al. Reference Maness, Kushner and Fung2013). Severe host depletion has even forced the beetle into traditional non-hosts of spruce Picea glauca (Moench) Voss×P. engelmannii Parry ex Engelmann in some areas (Huber et al. Reference Huber, Aukema, Hodgkinson and Lindgren2009; McKee et al. Reference McKee, Huber and Aukema2013). Of greater concern has been the gradual expansion of its geographic range in western Canada during the last several decades due to an increase in thermally benign habitat (Carroll et al. Reference Carroll, Taylor, Régniere and Safranyik2004). This is not unlike expansions seen in the United States of America into higher elevation five-needle pines (Logan and Powell Reference Logan and Powell2001; Weed et al. Reference Weed, Ayres and Hicke2013).
Range expansion by the mountain pine beetle has occurred northward (Sambaraju et al. Reference Sambaraju, Carroll, Zhu, Stahl, Moore and Aukema2012) and eastward over the northern Rocky Mountains (Robertson et al. Reference Robertson, Nelson, Jelinski, Wulder and Boots2009; de la Giroday et al. Reference de la Giroday, Carroll, Lindgren and Aukema2011, Reference de la Giroday, Carroll and Aukema2012) and across the Alberta Plateau (Janes et al. Reference Janes, Li, Keeling, Yuen, Boone and Cooke2014; Tsui et al. Reference Tsui, Farfan, Roe, Rice, Cooke and El-Kassaby2014). Infestations are now established within the region where lodgepole pine (Pinus contorta latifolia (Engelmann) Critchfield), the primary host of the beetle (Safranyik and Carroll Reference Safranyik and Carroll2006), intermingles and hybridises with jack pine (P. banksiana Lambert) (Cullingham et al. Reference Cullingham, Cooke, Dang, Davis, Cooke and Coltman2011), a suitable host for mountain pine beetle (Furniss and Schenk Reference Furniss and Schenk1969; Cerezke Reference Cerezke1995) and a major component of the North American boreal forest. There is grave concern that the insect could now move through the Canadian boreal forest to novel pine hosts of eastern North America (Cerezke Reference Cerezke1995; Lusebrink et al. Reference Lusebrink, Erbilgin and Evenden2013; Erbilgin et al. Reference Erbilgin, Ma, Whitehouse, Shan, Najar and Evenden2014). In a recent review of the invasive potential of the mountain pine beetle, Safranyik et al. (Reference Safranyik, Carroll, Régnière, Langor, Riel and Shore2010) concluded that continued eastward expansion into the boreal forest was a significant probability. However, lack of knowledge regarding altered trophic interactions in novel habitats precluded the capacity to predict the rate of spread and subsequent impacts of the invading population. This point is particularly relevant since new evidence suggests that the defensive response (Clark et al. Reference Clark, Carroll and Huber2010, Reference Clark, Pitt, Carroll, Lindgren and Huber2014), the ability of beetles to aggregate (Burke and Carroll Reference Burke and Carroll2016), and the ultimate productivity of the mountain pine beetle (Cudmore et al. Reference Cudmore, Bjorklund, Carroll and Lindgren2010), in putatively “evolutionarily naïve” trees may be very different from expectations derived from the beetle’s historic habitat.
A changing landscape in the epidemiology and population dynamics of bark beetles
Canada’s forested landscape today covers 348 million ha, still harbouring a wide variety of species of bark beetles from coast to coast (Swaine Reference Swaine1918; Bright Reference Bright1976). Defoliators such as spruce budworm (C. fumiferana) join native bark beetles as significant disturbance agents in Canada’s boreal forest (Royama Reference Royama1984; Hicke et al. Reference Hicke, Allen, Desai, Dietze, Hall and Hogg2012). Yet the template on which these biotic disturbance agents exist is under accelerating change (Bentz et al. Reference Bentz, Régnière, Fettig, Hansen, Hayes and Hicke2010; Gauthier et al. Reference Gauthier, Bernier, Burton, Edwards, Isaac and Isabel2014). Current rates of global change, manifested by introductions of invasive species and warming climates, could not have been anticipated in Swaine’s time.
The rates of introduction of invasive forest insect pests to North America are increasing (Work et al. Reference Work, McCullough, Cavey and Komsa2005; Aukema et al. Reference Aukema, McCullough, Von Holle, Liebhold, Britton and Frankel2010). While Swaine omitted other wood and bark boring families such as the Buprestidae and Cerambycidae, he could not have anticipated the impacts or mitigation efforts precipitated by the arrivals of Asian longhorned beetle (Anaplophora glabripennis (Motschulsky); Coleoptera: Cerambycidae), the emerald ash borer (Agrilus planipennis Fairmaire; Coleoptera: Buprestidae), or the brown spruce longhorn beetle (Tetropium fuscum (Fabricius); Coleoptera: Cerambycidae), to Canada over the past decade. Mercifully, no exotic bark beetles have had such pronounced effects on Canadian natural and urban forests to date, although the introduction of other invasive species has altered the population dynamics of some natives.
Swaine could not have foreseen that invasive species would cause some of the “neutral” species he highlighted to become threatened with extinction or synonymous with mass mortality within a century, for example. The eastern ash bark beetle (Hylesinus aculeatus (Say); Coleoptera: Curculionidae) was noted throughout the range of ash in eastern Canada (Swaine Reference Swaine1918). Now, the insect is listed at “high risk” due to the widespread mortality of ash (Fraxinus Linnaeus; Oleaceae) in North America caused by the emerald ash borer (Gandhi and Herms Reference Gandhi and Herms2010). On elms, the innocuous and native elm bark beetle H. rufipes was “not known to cause any injury to living trees”. At the time of Swaine’s work, a malady among elms had been noted in Europe but it was not until the 1920s that the Dutch elm disease pathogen Ophiostoma ulmi (Buisman) Melin and Nannfeldt (Ophiostomataceae) was identified (Brasier and Buck Reference Brasier and Buck2001). In ensuing years this pathogen arrived in North America with another bark beetle vector identified as Scolytus multistriatus (Marsham). Now inextricably linked with a subsequent virulent pathogen Ophiostoma novo-ulmi Brasier in the complex, H. rufipes unfortunately can no longer be considered a neutral species as Swaine had it placed (Collins et al. Reference Collins, Buchanan, Whitten and Hoffmann1936; Anderson and Holliday Reference Anderson and Holliday2003).
Likewise, Swaine probably did not give thought to native bark beetles of Canada that might eventually display new dynamics in other regions of the world. The red turpentine beetle (Dendroctonus valens LeConte) was listed as an “important assistant” of mountain pine beetle in ponderosa pine (Pinus ponderosae Douglas ex Lawson), where it could kill “patches of bark at the base of living pines and spruces” (Swaine Reference Swaine1918). In the 1980s, this insect was accidentally introduced from North America to China, where it is now an aggressive tree-killing bark beetle on pines devoid of any coevolutionary association (Yan et al. Reference Yan, Sun, Don and Zhang2005; Sun et al. Reference Sun, Lu, Gillette and Wingfield2013). There is now grave concern the beetle could return to North America with novel fungi, and create a similar pandemic on pines here (Lu et al. Reference Lu, Zhou, De Beer, Wingfield and Sun2009; Taerum et al. Reference Taerum, Duong, de Beer, Gillette, Sun and Owen2013).
Conclusion: advice from the past
Despite stark differences in environmental conditions between the turns of the 20th and 21st centuries that affect bark beetle population dynamics, we conclude by noting two adages from Swaine related to management strategies that still hold true for today and into the future. First, despite the broad generalisations in places in his text, Swaine reminded the reader that each management scenario is unique, as each prescription for control depends “upon the species of beetles, and partly upon local conditions”. Richmond (Reference Richmond1986) elaborated that forest ecologists should “not confuse an issue in eastern North America with a similar problem in the west. While the species of insect may be the same, most other factors will be totally different”. Who could have known such a statement would be so apropos in researching solutions to range expansions of mountain pine beetle, unforeseen a century ago?
Familiarize yourself with your predecessors’ mistakes, and don’t repeat them. Don’t be ashamed of your mistakes – profit by them (H.A. Richmond Reference Richmond1986).
While the first recommendation advocates for context-dependent management, it prompts the question of what recommendations are due in each situation. Hence, Swaine’s second adage most critically stipulated that all activities “should be undertaken under the direction of a competent forest entomologist”. Swaine felt strongly about training the next generation of forest health professionals; in fact, Swaine taught one of the first entomology courses in Canada at Macdonald College with Professor William Lockhead in 1907 (Johnstone Reference Johnstone1991). Canada has benefited from an exceptionally rich heritage of bark beetle ecologists in addition to Swaine over the past century: Richmond, Hopping, McCambridge, Reid, Shepherd, Whitney, Safranyik, Shore, de Groot, Borden, Lindgren, and others. A synthesis of their accomplishments is beyond the scope of this work, and perhaps not even appropriate: Richmond encouraged future generations to dwell on their forerunners’ foibles. “Familiarize yourself with your predecessors’ mistakes, and don’t repeat them. Don’t be ashamed of your mistakes – profit by them” (Richmond Reference Richmond1986). This collaborative article was authored by a duo of graduate students and their professors. With accelerating rates of species invasions and changing climates, the need for well-qualified forest managers will not diminish in the next century.
Acknowledgements
The authors are indebted to a global bark beetle community that extends beyond Canada and continues to make noteworthy advances in bark beetle ecology in the face of a rapidly changing world. Given the principle nanos gigantum humeris insidentes (discovering truth by building on previous discoveries), they regret that many worthy papers are not cited in this review. The authors appreciate the editorial assistance of A.M. Wilke (University of Minnesota) and the assistance finding the only known photograph of J.M. Swaine by M.M. Furniss (United States Forestry Service retired; University of Idaho). Helpful comments on previous versions of this work were provided by D. Langor and R. Alfaro (Canadian Forest Service), D. Rosenberger (University of Minnesota), and two anonymous reviewers. Thanks to Les Safranyik for sharing his knowledge of spruce beetle natural history. Funding for this work was provided in part by United States Forest Service Evaluation Monitoring project NC-EM-B-12-01, the University of Minnesota Agricultural Experimental Station, and the British Columbia Future Forest Ecosystems Science Council.




