Introduction
Phoresy is a dispersal strategy, whereby the dispersing organism (the phoront) associates with another organism (the host) to move between habitats. Many species of mites, which are small, wingless, and often associated with ephemeral resources, depend on a phoretic lifestyle with larger, more mobile organisms. The presence of life stages with anatomical and behavioural modifications that are specialised for phoresy is common in mite taxa (Bartlow and Agosta Reference Bartlow and Agosta2021; Seeman and Walter Reference Seeman and Walter2023). Phoretic mites can engage in complex interactions with their hosts and other symbionts and ultimately can impact the population dynamics of their hosts.
Beetles (Coleoptera) host a particularly high diversity of phoretic mites (Seeman and Walter Reference Seeman and Walter2023). Because of many bark beetles’ (Curculionidae: Scolytinae) economic importance, the symbionts, including mites, of some beetle species have been relatively well studied. The nature of the ecological relationship between mites and their bark beetle hosts can range from beneficial to harmful. For example, Proctolaelaps dendroctoni Lindquist and Hunter, which is phoretic on the southern pine beetle, Dendroctonus frontalis Zimmerman, is also a predator of the beetle larvae and pupae and has been identified as potential biological control agent (Moser Reference Moser1975). In some cases, predatory mites may benefit their phoretic hosts by feeding on the hosts’ natural enemies (Hofstetter et al. Reference Hofstetter, Knee and Khaustov2023). Some species of Tarsonemus (Trombidiformes: Tarsonemidae) have structures called sporothecae, in which the mites carry ascospores of fungi that may compete with fungi carried in the mycangia of their bark beetle hosts, which the beetles use to feed their larvae. The presence of Tarsonemus mites has been suggested to result in population declines in southern pine beetles (Moser Reference Moser1985; Lombardero et al. Reference Lombardero, Ayres, Hofstetter, Moser and Lepzig2003; Hofstetter et al. Reference Hofstetter, Klepzig, Moser and Ayres2006). Other studies suggest that mite loads may negatively impact the flight and mobility of their beetle hosts (Atkins Reference Atkins1960; Rocha et al. Reference Rocha, Pozo-Velázquez, Faroni and Guedes2009).
The mite assemblage of the Douglas-fir beetle, Dendroctonus pseudotsugae Hopkins (Coleoptera: Curculionidae), remains poorly documented because most studies on bark beetle phoronts focus on more economically important species, such as D. frontalis and Ips typographus Linnaeus (Coleoptera: Curculionidae) (Hofstetter et al. Reference Hofstetter, Moser and Blomquist2014, Reference Hofstetter, Dinkins-Bookwalter, Davis, Klepzig, Vega and Hofstetter2015). The Douglas-fir beetle attacks mainly Douglas-fir trees, Pseudotsuga menziesii (Mirbel) Franco (Pinaceae), and occasionally western larch, Larix occidentalis Nuttall (Pinaceae) (Kelley and Farrell Reference Kelley and Farrell1998). Adult Douglas-fir beetles create galleries and lay eggs in the phloem of their host trees, where offspring feed and develop into adults (Stark Reference Stark, Mitton and Sturgeon1982). Douglas-fir beetles also inoculate the trees they attack with fungi that, among other things, block the host trees’ defences and can lead to tree death when beetle abundances are sufficiently high (Raffa et al. Reference Raffa, Aukema, Bentz, Carroll, Hicke, Turner and Romme2008). The Douglas-fir beetle can be a major economic concern across its range because it is capable of killing large numbers of hosts during outbreaks. For instance, in 2021, the Douglas-fir beetle was the third-most active bark beetle in British Columbia, Canada, affecting over 106 000 ha of forested land in the province (BC Ministry of Forests, Lands, Natural Resource Operations and Rural Development 2021). Irruptive population growth and epidemic-level outbreaks can also occur in combination with other forest disturbances, and Douglas-fir beetle infestations may become more extensive with climate change (Bentz et al. Reference Bentz, Régnière, Fettig, Hansen, Hayes and Hicke2010; Cole et al. Reference Cole, Andrus, Butkiewicz, Rodman, Santiago and Tutland2022). By documenting the phoretic mite assemblage of the Douglas-fir beetle, we gain a greater understanding of the diversity of organisms associated with this species and provide a basis for further study of the mites’ roles in the beetle’s population dynamics.
In the present study, we used a combination of morphological and genetic analyses to describe the taxonomic diversity of the phoretic mite assemblage of the Douglas-fir beetle in central British Columbia. We also examined mite loads in relation to host beetles’ sex and emergence phenology. To our knowledge, this study is one of the first performed on the phoretic mite assemblage of Douglas-fir beetles.
Methods
We sampled Douglas-fir beetles from May to August 2021 and 2022 in forested areas around the University of Northern British Columbia, Prince George, British Columbia (53.8922° N, 122.8134° W). The campus is situated in the sub-boreal spruce (SBS) ecozone and the dry–warm subzone (SBSdw2) of British Columbia, characterised by mixed stands of lodgepole pine (Pinus contorta Douglas ex Loudon (Pinaceae)), Douglas-fir, and interior hybrid spruce (Picea engelmannii f. glauca (Richard Smith) Beissner (Pinaceae)). In both years, 12-unit Lindgren multiple funnel traps (Lindgren Reference Lindgren1983) baited with a commercially available Douglas-fir beetle pheromone trap blend (standard lure, enhanced, catalogue #3187, Synergy Semiochemicals Corporation, Burnaby, British Columbia) were used for sampling. In 2021, two traps were placed at each of three sites, and no pesticide was used in the trap cups. In 2022, pest strips were required to stop the predation on Douglas-fir beetles by checkered beetles (Cleridae) and to gather an accurate count of the beetles captured in each trap. However, we observed that the contact-insecticide cubes (cut from Vapona No-pest Strip, Fisons Horticulture Inc. Mississauga, Ontario, Canada) killed some Douglas-fir beetles with their elytra spread open, which could have resulted in the loss of mites attached to the subelytral surface. Therefore, in 2022, two traps were placed at seven locations around the campus: one trap at each site contained a small pesticide-infused polymer block and the other trap did not. Beetles collected from traps containing pesticide were counted to determine emergence phenology, whereas beetles collected from traps not containing pesticide were inspected for mites. In both years, the traps were checked two to four times per week, and all live beetles from which mites were sampled were individually collected in microcentrifuge tubes containing approximately 1.5 mL of 95% ethanol to decrease the likelihood of including mites associated with other insects in our collection. Dead beetles in the traps containing pesticide in 2022 were collected into sealable plastic bags.
The sex of the beetles was determined through the presence or absence of the dorsal abdominal stridulating organ that males use to create acoustic signals (Lyon Reference Lyon1958). Following the confirmation of the sex, the elytra were removed for inspection, and the entire body of each beetle was inspected for mites, which were then removed, counted, and categorised into morphospecies by features such as size, body shape, unique features, and colouration. Mites suspended in the ethanol were included in the count because beetles that were inspected for mites were collected individually into ethanol, so mites in the ethanol were likely associated with the beetles. The mite assemblage of each beetle was stored separately from its corresponding host (also stored individually) in 95% ethanol. Representative mites of each of the morphospecies that were identified were chosen for DNA barcoding.
The cytochrome c oxidase subunit 1 (CO1) DNA barcoding regions of 94 mites associated with Douglas-fir beetles were successfully sequenced at the Biodiversity Institute of Ontario (University of Guelph, Guelph, Ontario, Canada), and sequence data were used to group them into operational taxonomic units (OTUs), each of which was given an identifying letter (e.g., OTU A). Sequences greater than 400 base pairs and with no contaminants or stop codons were considered successful and used in the analysis. A total of 33 beetle specimens were also barcoded at the Biodiversity Institute of Ontario, all of which were matched to existing Douglas-fir beetle sequences (matched at greater than 99.84%). All mite and beetle specimens were vouchered at the Biodiversity Institute of Ontario, and barcode data are accessible in dataset DS-DFBMITES. Operational taxonomic unit clustering was performed using the Barcode of Life Database (BOLD) dashboard with the BOLD aligner using MUSCLE (Edgar Reference Edgar2004; Ratnasingham and Hebert Reference Ratnasingham and Hebert2007).
In 2023, intact voucher individuals of all OTUs except for OTU H (which were not available) were sent to H.C.P. at the University of Alberta (Edmonton, Alberta, Canada) for morphological identification. Large-bodied mites were placed in 80% lactic acid for 24 hours to clear before mounting, but many of the mites were extremely small and did not require clearing. The mites were mounted on glass slides in commercially prepared polyvinyl alcohol mounting medium (BioQuip Products, Rancho Dominguez, California, United States of America) and covered with 15-mm round cover slips. Slides were then cured for four days on slide warmers set at 40 °C. Mounted mites were examined using a Leica DMLB compound microscope (Leica Camera AG, Wetzlar, Germany) with differential interference contrast lighting and were identified using the relevant taxonomic literature (Chant Reference Chant1963; Woodring Reference Woodring1966; Krantz and Walter Reference Krantz and Walter2009; Skvarla et al. Reference Skvarla, Fisher and Dowling2014; Khaustov et al. Reference Khaustov, Klimov, Trach, Bobylev, Salavatulin, Khaustov and Tolstikov2018). Slide-mounted specimens have been deposited in the E.H. Strickland Entomological Museum at the University of Alberta (accession numbers UASM407380 to UASM407395). Images used for identification, with file names indicating OTUs (Table 1), are available from Browning et al. (Reference Browning, Proctor and Huber2024).
Table 1. Potential taxonomic matches based on DNA barcoding and morphological identification of slide-mounted vouchers and prevalence of mite operational taxonomic units (OTUs) A–J from the 2021 and 2022 collections. No DNA barcodes are available for OTUs G, I, and J. Prevalence is the percent of Douglas-fir beetle hosts that had at least one mite of a specific OTU on their body. Abundance is the mean number of mites per beetle for an OTU. Due to similar morphology, OTUs B1 and B2 were combined in 2021 analysis but were separated in 2022 when it became clear they were separate OTUs. BOLD, Barcode of Life Data System; NCBI, National Center for Biotechnological Information
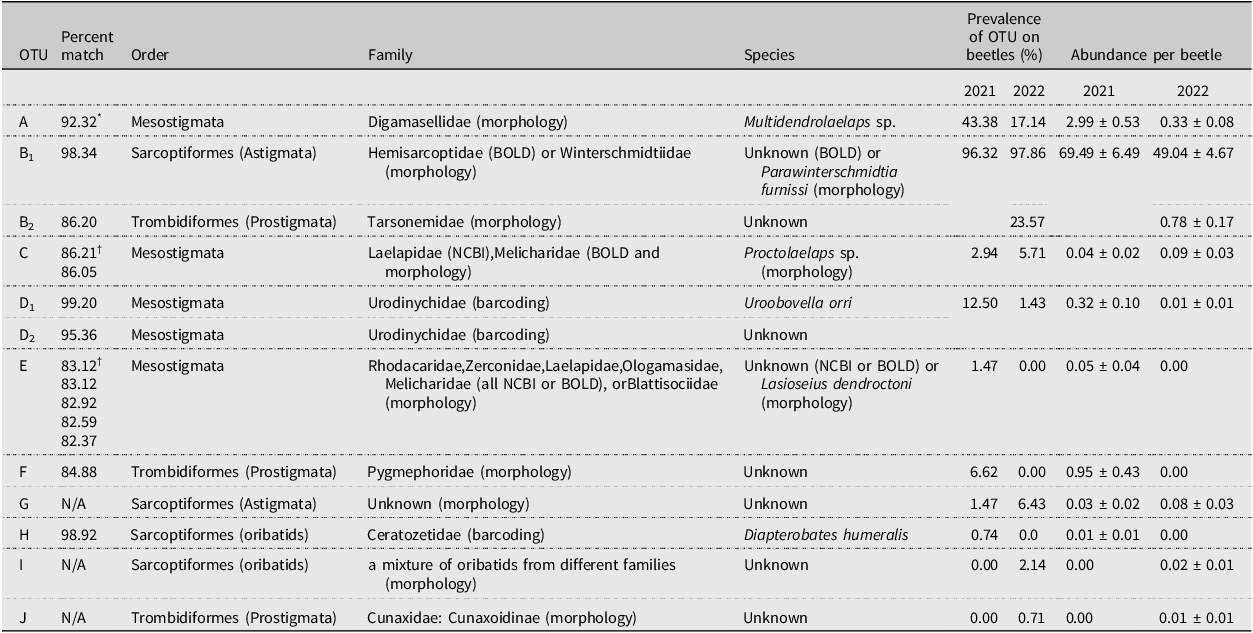
* Initial Barcode of Life Database (BOLD) match for OTU A was a 92.32% match to Rhodacaridae; however, subsequent morphological analysis by Babaeian determined the identity of OTU A as Digamasellidae: Multidendrolaelaps sp.
† Matches to entries at the National Center for Biotechnological Information database. All other matches are to specimens in BOLD.
The prevalence (proportion of beetles carrying each OTU) and abundance (mean number of a specific OTU per beetle) of each mite OTU were recorded. A Spearman’s rank correlation test was used to assess the association between the number of beetles caught in the traps and the number of mites categorised in each OTU. This analysis was performed only for the samples caught until 10 July 2022 because the majority of beetle flight was captured in that time period. Only OTUs A–C were used in the present analysis because, compared to the other OTUs, only OTUs A–C were abundant enough to be found throughout most of the trapping season. A Mann–Whitney U test was used to assess if the number of associated OTUs A–F and H carried by each sex differed significantly between male and female host beetles. Nonparametric tests were used in this study because the data did not follow a bivariate normal distribution. All statistical analyses were performed in R, version 2022.12.0+353, with an alpha level of 0.05.
Results
Molecular identification of operational taxonomic units
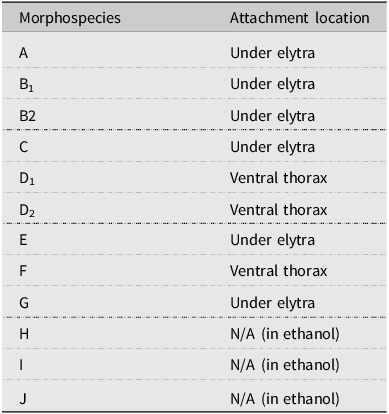
We counted a total of 10 047 mites from 137 beetles in 2021 and 7045 mites from 141 beetles in 2022. We initially identified 10 morphospecies that were found attached either underneath the elytra or on the ventral surface of the thorax (morphospecies A–G; Table 2). In three instances (morphospecies H–J), the mites were found only in the storage ethanol but were included in the analysis for completeness. The DNA barcoding process distinguished nine OTUs (> 2% sequence divergence), differing slightly from the morphospecies analysis but mostly supporting the morphological groupings. Barcoding repeatedly failed for morphospecies G, I, and J, whereas morphospecies B was found to include two distinct OTUs (hereafter referred to as OTUs B1 and B2). This allowed us to recognise the morphological differences between the two, and the counts of B1 and B2 were separated in 2022. Morphospecies D was found to be two distinct but related OTUs (hereafter referred to as OTUs D1 and D2), but their counts were not separated in 2022 due to substantial morphological similarity that made distinguishing between them unreliable. Only counts from OTUs confirmed by DNA barcoding were included in the prevalence, abundance, and phenological data analyses.
Table 2. Mite morphospecies associated with the Douglas-fir beetles and the attachment locations on the insect hosts
The successfully barcoded specimens were assigned to three taxonomic orders by barcode matches: Mesostigmata (OTUs A, C, D1, D2, and E), Trombidiformes (OTUs B2 and F), and Sarcoptiformes (OTUs B1 and H; Table 1). Most OTUs were associated with a single taxonomic family, based on the closest BOLD match; however, OTUs C and E were similar to multiple families, as is shown in Table 1. OTUs D1 and H were the only OTUs identified by BOLD matches to species: these were Uroobovella orri Hirschmann (Mesostigmata: Urodinychidae) and Diapterobates humeralis Hermann (Sarcoptiformes: Ceratozetidae), respectively.
Morphological identification of operational taxonomic units
Slide-mounted exemplars that were morphologically identified by H.C.P. did not always match the BOLD identifications (Table 1). Family-level identifications were supported for OTUs B2 (Tarsonemidae) and F (Pygmephoridae), and they were corroborated at a higher taxonomic rank for OTU D (Uropodina). Three OTUs without DNA barcodes were identified morphologically by H.C.P. and Lisa Lumley (Alberta Biodiversity Monitoring Institute, Edmonton, Alberta): these were OTU G = deutonymphal Astigmata, OTU I = two species of oribatids (Cymbaeremaeidae: Scapheremaeus palustris (Sellnick), the other possibly a member of the Oripodidae), and OTU J = Cunaxidae: Cunaxoidinae sp. Operational taxonomic unit G is a visual match with Schweibea nova (Oudemans) (Acaridae) in Khaustov et al. (Reference Khaustov, Klimov, Trach, Bobylev, Salavatulin, Khaustov and Tolstikov2018), fig. 22C, D; however, as the specimens mounted in the present study were not taken through a key, we are not confident that OTU G is indeed a Schweibea species. Two OTUs for which there were multiple barcode matches at the family level were morphologically identified to a single family each: these were OTU C = Melicharidae: Proctolaelaps sp. and OTU E = Blattisociidae: Lasioseius dendroctoni Chant. The morphological identification of OTU A (Digamasellidae) by H.C.P. matched that by Esmaeil Babaeian (University of Tehran, Tehran, Iran), which contradicted the BOLD identification of Rhodacaridae (see Table 1, footnote). Perhaps most importantly, the numerically dominant OTU B1, identified as Hemisarcoptidae by the BOLD match, was morphologically identified by H.C.P. as Winterschmidtiidae: Parawinterschmidtia furnissi (Woodring) (Browning et al. Reference Browning, Proctor and Huber2024). It is possible that the beetles hosted deutonymphs of both mite families and, by chance, only the hemisarcoptids were sent for barcoding and only the winterschmidtiids were sent to H.C.P. for slide-mounting. A photo of an extracted specimen of OTU B1 at BOLD (specimen UNBC1-35-04 in DS-DFBMITES) is morphologically consistent with P. furnissi; however, deutonymphal winterschmidtiids and hemisarcoptids are morphologically quite similar, so without slide-mounted BOLD–extracted specimens, we cannot confirm the photo is of P. furnissi.
Relative and absolute abundance
Of all the OTUs, OTU B1 was by far the most prevalent and abundant (Table 1). Operational taxonomic unit B1 mites were consistently found grouped, often in very high numbers, in an anterior pocket on the beetles’ subelytral surface (Fig. 1). In 2022, a mean of 50.5 ± 4.7 mites per beetle was observed; however, when OTU B1 was removed from the analysis, the mean was only 1.3 ± 0.2 mites per beetle. Most beetles carried between 1 and 50 (54.9%) mites, and a smaller number of beetles carried 50–150 (33.7%) mites. Beetles were less likely to carry 150–300 mites (10.2%), and a very small portion of beetles carried 300–500 mites (1.2%). One beetle carried 456 mites, which was the maximum number we observed. Alternatively, without OTU B1 in the analysis, the majority of beetles carried 0–2 (84.8%) mites, and a smaller number of beetles carried 2–14 (15.2%) mites.

Figure 1. Mites belonging to operational taxonomic unit (OTU) B1, clustered at the top of both images, at the anterior end of the elytra, and a single representative of OTU A, seen in the middle of the elytron in the left image, found on the subelytral surface from two representative Douglas-fir beetles collected in the present study.
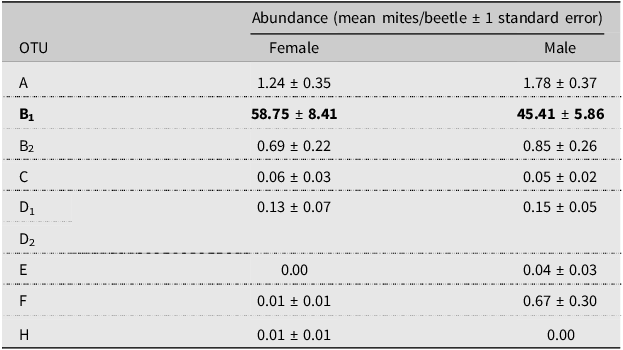
Mite abundance varied throughout our sampling period (Fig. 2). Operational taxonomic units A and B2 were negligibly and positively correlated with beetle numbers, whereas OTU B1 was moderately and negatively correlated, and OTU C was weakly and positively correlated with the number of beetles caught (OTU A Spearman R = 0.141, P = 0.630; OTU B1 Spearman R = –0.410, P = 0.151; OTU B2 Spearman R = 0.090, P = 0.770; and OTU C Spearman R = 0.253, P = 0.382). However, the high P-values associated with each Spearman’s coefficient indicate the relationships could be due to chance. The mite loads of most OTUs carried by male and female beetles did not differ (OTU B2 n = 36 females and 95 males; OTUs A, C, D1, D2, E, F, H n = 70 females and 191 males; Mann–Whitney P = 0.102 – 0.678; Table 3). Operational taxonomic unit B1 was significantly differentially abundant between male and female beetles, with female beetles having significantly more associated OTU B1 mites (n = 36 females and 95 males; Mann–Whitney P = 0.0336; Table 3).
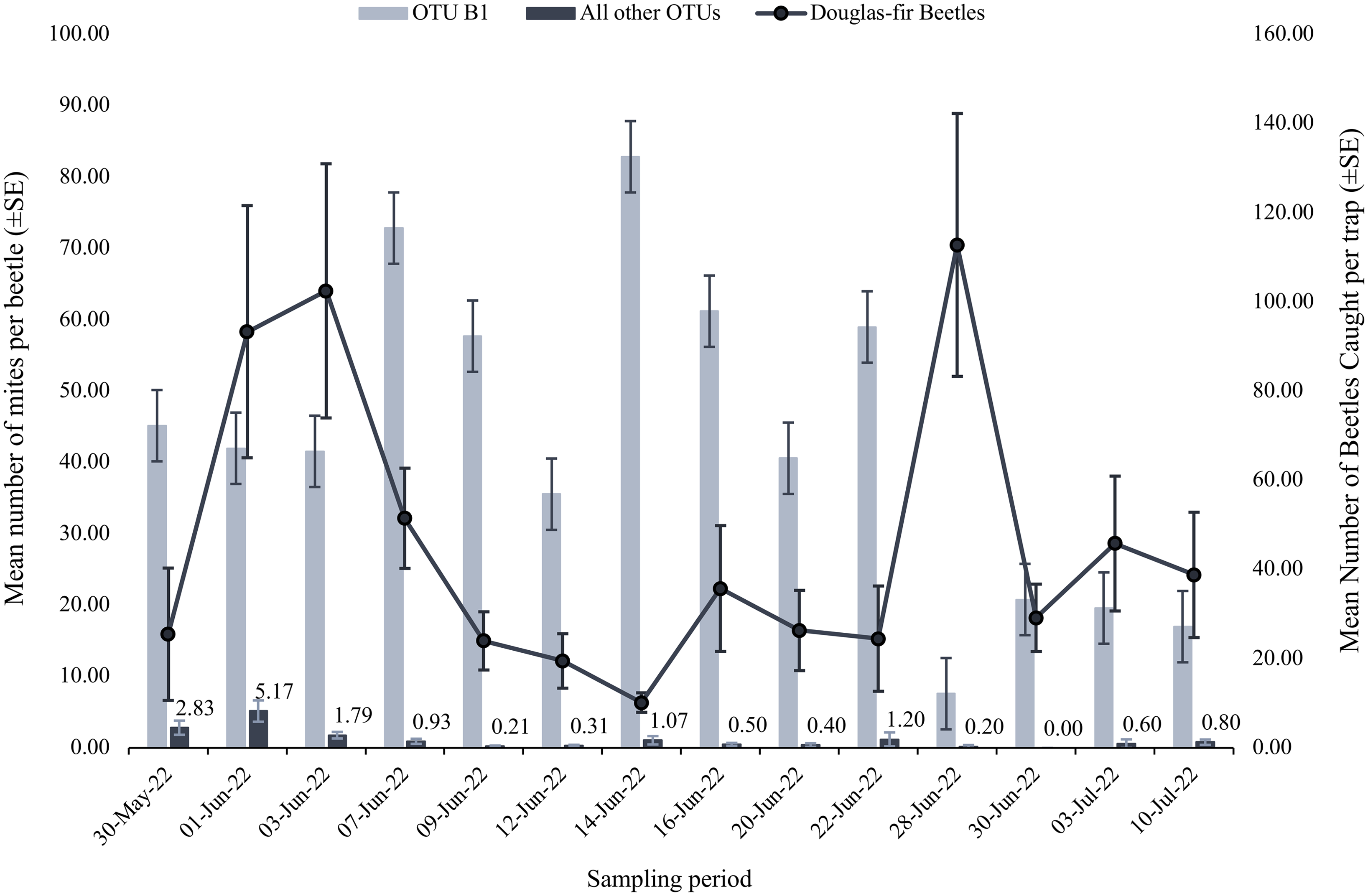
Figure 2. Mean number (± 1 standard error) of operational taxonomic unit (OTU) B1 (light blue) and all other OTUs (dark blue) per Douglas-fir beetle (y-axis on left) and the mean number of Douglas-fir beetles (± 1 standard error) collected on each collection date between 30 May 2022 and 10 July 2022 (y-axis on right).
Table 3. Abundance (mean number of mites per beetle) of each operational taxonomic unit (OTU) per female and male Douglas-fir beetles. Operational taxonomic unit B1 (in bold) was found in significantly different abundances between beetle sexes and was found in higher numbers on female beetles
Discussion
At least nine distinct mite OTUs were associated with Douglas-fir beetle hosts, as determined by DNA barcoding. With the additional four taxa that were not successfully barcoded but were identified morphologically (Table 1), up to a dozen phoretic mite species were found to be associated with the Douglas-fir beetles in the present study. Of the barcoded specimens, most were found to have BOLD and/or morphological matches to families that contain species associated with other bark beetle species. Here, we discuss OTUs in order of their listing in Table 1.
Family Digamasellidae (Mesostigmata) contains Multidendrolaelaps Hirschmann spp., which are morphologically closely associated with Dendrolaelaps Halbert spp. (e.g., Lindquist Reference Lindquist1975; Huhta and Karg Reference Huhta and Karg2010). Dendrolaelaps spp. are known phoronts of several bark beetle species, including D. frontalis, D. ponderosae, I. typographus, and Ips confusus LeConte, and are predators of multiple life stages of their phoretic hosts (Moser Reference Moser1975; Khaustov et al. Reference Khaustov, Klimov, Trach, Bobylev, Salavatulin, Khaustov and Tolstikov2018; Vissa et al. Reference Vissa, Mercado, Malesky, Uhey, Mori and Knee2020; Hofstetter et al. Reference Hofstetter, Knee and Khaustov2023). Operational taxonomic unit B1 was identified as a member of the astigmatan family Hemisarcoptidae, based on barcoding, but as Winterschmidtiidae, based on morphology of slide-mounted specimens. This may indicate that, by chance, members of one family were sent for barcoding and others were sent for slide-mounting, or that errors occurred in the identification of specimens whose sequences are in BOLD. Members of both families are associated with bark beetles. For example, Nanacarus Oudemans spp. (Hemisarcoptidae) are associated with tree bark, woody bracket fungi, and wood-boring insect galleries and have been found to form phoretic associations with I. typographus and D. frontalis (Penttinen et al. Reference Penttinen, Viiri and Moser2013; Hofstetter et al. Reference Hofstetter, Moser and Blomquist2014; Khaustov et al. Reference Khaustov, Klimov, Trach, Bobylev, Salavatulin, Khaustov and Tolstikov2018). Woodring (Reference Woodring1966) recorded Parawinterschmidtia furnissi (Winterschmidtiidae) from D. pseudotsugae from Idaho (originally described as Calvolia furnissi). The Tarsonemidae is a fungivorous group that contains members with fungal spore-carrying sporothecae (Moser Reference Moser1985). Numerous different Tarsonemus spp. are phoretic on I. typographus, D. ponderosae, and D. frontalis (Mori et al. Reference Mori, Proctor, Walter and Evenden2011; Hofstetter et al. Reference Hofstetter, Moser and Blomquist2014; Mercado et al. Reference Mercado, Hofstetter, Reboletti and Negrón2014; Khaustov et al. Reference Khaustov, Klimov, Trach, Bobylev, Salavatulin, Khaustov and Tolstikov2018; Vissa et al. Reference Vissa, Mercado, Malesky, Uhey, Mori and Knee2020). Proctolaelaps A. Berlese spp. (Mesostigmata: Melicharidae) and Androlaelaps casalis A. Berlese (Mesostigmata: Laelapidae) are also common associates of I. typographus, I. confusus, D. ponderosae, and D. frontalis (Moser Reference Moser1975; Mori et al. Reference Mori, Proctor, Walter and Evenden2011; Khaustov et al. Reference Khaustov, Klimov, Trach, Bobylev, Salavatulin, Khaustov and Tolstikov2018; Chaires-Grijalva et al. Reference Chaires-Grijalva, Estrada-Venegas, Quiroz-Ibáñez, Equihua-Martínez, Moser and Blomquist2019; Vissa et al. Reference Vissa, Mercado, Malesky, Uhey, Mori and Knee2020; Hofstetter et al. Reference Hofstetter, Knee and Khaustov2023). Proctolaelaps spp. and A. casalis have been observed to eat the eggs, larvae, and pupae of their associated bark beetles (Moser Reference Moser1975). Mites in family Urodinychidae have a deutonymphal life stage dedicated to phoresy that attaches to hosts (including bark beetles) via a secreted anal pedicel (Knee et al. Reference Knee, Beaulieu, Skevington, Kelso, Cognato and Forbes2012a); the putative Urodinychidae in this study (OTUs D1 and D2) also exhibited an anal pedicel. The species match of OTU D1, Uroobovella orri, has been reported as a phoretic host generalist and is associated with multiple species of bark beetles, including the Douglas-fir beetle (Knee et al. Reference Knee, Beaulieu, Skevington, Kelso, Cognato and Forbes2012a). Barcodes of OTU E were matched in BOLD at a low percentage to several families of Mesostigmata, but the one specimen that was slide-mounted for morphological identification was identified as Lasioseius dendroctoni (Blattisociidae). This species was described based on specimens associated with a Dendroctonus sp. from Corvallis, Oregon, United States of America, by Chant (Reference Chant1963), who noted that it was sufficiently distinctive due to the female’s fragmented sternal shield (Browning et al. Reference Browning, Proctor and Huber2024) that he considered erecting a new genus for it. Pygmephoridae is a fungivorous family, and certain females (phoretomorphs) have enlarged tarsal claws on their first legs to facilitate phoretic dispersal (Moser and Cross Reference Moser and Cross1975; Walter and Proctor Reference Walter and Proctor2013). Species of Elattoma Mahunka (Trombidiformes: Pygmephoridae) have been found associated with Ips calligraphus (Germar) and I. typographus (Khaustov et al. Reference Khaustov, Klimov, Trach, Bobylev, Salavatulin, Khaustov and Tolstikov2018; Chaires-Grijalva et al. Reference Chaires-Grijalva, Estrada-Venegas, Quiroz-Ibáñez, Equihua-Martínez, Moser and Blomquist2019). Based on morphological identification, OTU G is a deutonymphal Astigmata that matches the bark beetle–associated Schweibea nova (Oudemans) in Khaustov et al. (Reference Khaustov, Klimov, Trach, Bobylev, Salavatulin, Khaustov and Tolstikov2018, fig. 22C, D); however, because we did not have keys to the genera and species of acarids, we are not confident of this identification for our specimens. The species match of OTU H, the oribatid mite Diapterobates humeralis, has also been found in pheromone traps of bark beetles, but whether they are phoretic is unconfirmed (Penttinen et al. Reference Penttinen, Viiri and Moser2013; Cordes et al. Reference Cordes, Maraun and Schaefer2022). If they are, it is likely over short distances and in response to shifting abiotic conditions (Penttinen et al. Reference Penttinen, Viiri and Moser2013; Cordes et al. Reference Cordes, Maraun and Schaefer2022). The occurrence of two other species of oribatids (OTU I) and one species of Cunaxidae – predatory mites not often observed to be phoretic on bark beetles – may reflect accidental bycatch.
Compared to previous studies on other Dendroctonus spp., the mean number of mites found per Douglas-fir beetle in this study (∼50.5) was quite high. For example, the mean number of mites per individual D. frontalis was found to be 4.10 and 3.96 on females and males, respectively (Moser Reference Moser1976). Vissa et al. (Reference Vissa, Mercado, Malesky, Uhey, Mori and Knee2020) found mean mite numbers on D. ponderosae between 0.88 and 5.50 mites per beetle, whereas Hofstetter et al. (Reference Hofstetter, Knee and Khaustov2023) found an average of 18 mites per beetle on recently emerged I. confusus. Without the hyperabundant OTU B1, which was found exclusively under the anterior portion of the elytra, the mean number of mites found in the present study falls much closer to that recorded those other studies (∼1.3; e.g., Vissa et al. Reference Vissa, Mercado, Malesky, Uhey, Mori and Knee2020; Hofstetter et al. Reference Hofstetter, Knee and Khaustov2023).
Studies conducted on Hemisarcoptes Lignières (Sarcoptiformes: Hemisarcoptidae), a potential relative of OTU B1, may shed light onto the relationship between Douglas-fir beetles and the hyperabundant OTU B1. All nonphoretic life stages of Hemisarcoptes are predators of scale insects (Hemiptera: Diaspididae), but in their phoretic deutonymphal stage, the mites lack functional mouthparts and have setae modified as ventral suckers to attach to the subelytral surface of their Chilocorus Leach spp. (Coleoptera: Coccinellidae) hosts. Houck (Reference Houck, Bruin, van der Geest and Sabelis1999) reported the distribution of Hemisarcoptes under their host elytra and noted that they were nonrandomly attached on the epipleural margin of the subelytral surface and occurred in very high numbers (400–800 per beetle), similar to the nonrandom distribution and abundance of OTU B1 observed in the present study. Houck (Reference Houck, Bruin, van der Geest and Sabelis1999) related the attachment patterns of Hemisarcoptes to the avoidance of spines along the host’s subelytral surface, which the author hypothesised could damage the ventral surface of Hemisarcoptes. There are limited studies on the presence of subelytral spines in bark beetles, but Houck (Reference Houck, Bruin, van der Geest and Sabelis1999) did note that unspecified members of Curculionidae had subelytral spines. Irrespective of whether OTU B1 is a member of the Hemisarcoptidae or is rather P. furnissi, the aggregation of this one species of mites in a consistent location under the elytra in most of the beetles examined in the present study may indicate some form of symbiosis between these organisms.
Throughout the collection period, Douglas-fir beetles emerged in highest numbers in late May and early June and subsequently declined until increasing in numbers on a single date near the end of the collection periods (Fig. 2). Operational taxonomic unit B1 was most numerous on Douglas-fir beetles during much of the collection period, before sharply declining on the late-emerging beetles (Fig. 2). Other OTUs were most abundant on the early-flying beetles, before mostly declining throughout the rest of the sampling period (Fig. 2). Previous studies have noted that mite abundances tended to peak at similar times as the flight peaks of their bark beetle hosts and during the highest abundances of their coleopteran hosts (Paraschiv et al. Reference Paraschiv, Martínez-Ruiz and Fernández2018; Knee et al. Reference Knee, Hartzenberg, Forbes and Beaulieu2012b). This is mostly supported in the present study by OTUs A, B2, and C being negligibly and moderately positively correlated with the number of beetles. Hofstetter et al. (Reference Hofstetter, Knee and Khaustov2023) found that species within families Digamasellidae and Tarsonemidae (related to OTUs A and B2) were most abundant on early-emerging beetles. Interestingly, OTU B1 was moderately negatively correlated with beetle numbers, indicating it may have an adverse impact on Douglas-fir beetle populations. However, both positive and negative relationships were weak and nonsignificant, and the patterns are unlikely to be biologically relevant.
All except one OTU were found equally on male and female beetles. Knee et al. (Reference Knee, Hartzenberg, Forbes and Beaulieu2012b) hypothesised that, on cerambycid beetles, due to a female beetle’s proximity to the egg niche where males do not enter, mites may have an advantage entering the beetle’s gallery when associated with a female. However, in the case of the Douglas-fir beetle, both males and females construct the egg gallery, and therefore, mites attached to either sex would have equal opportunity to enter the tree. This finding is supported by other studies of the potential sex bias of phoretic mites on other bark beetles, which showed no sex bias (Moser Reference Moser1976; Mori et al. Reference Mori, Proctor, Walter and Evenden2011; Paraschiv et al. Reference Paraschiv, Martínez-Ruiz and Fernández2018). That being said, OTU B1 was found in significantly higher numbers on female Douglas-fir beetles. Houck (Reference Houck, Bruin, van der Geest and Sabelis1999) noted that larger body sizes in ladybird beetles (Coccinellidae) provided more space for mite attachment. Douglas-fir beetle females are generally larger than males, and higher numbers of the most prevalent mite may simply be the result of more attachment space.
The present study is one of the first to document the diversity, phenology, host preference, and attachment locations of the phoretic mite assemblage of the Douglas-fir beetle. The mite assemblage includes at least nine distinct OTUs from multiple different taxonomic families with unique life histories and potential impacts on the Douglas-fir beetle. Particularly, we hypothesise the potential importance of the hyperabundant OTU B1 to Douglas-fir beetles during their adult tree colonisation phase, and we suggest that this phenomenon deserves further research in this insect and in analogous situations with other Curculionidae.
Acknowledgements
We thank Mackenzie Howse, Claire Paillard, and Anne Robinson, University of Northern British Columbia, along with Margaret Browning, Steve Browning, and Chantal Presse for their help in the field. We thank Drs. Aija White and Heather Bryan, University of Northern British Columbia, for their invaluable discussions and advice throughout the study. We are also grateful for the assistance of Dr. Esmaeil Babaeian, University of Tehran, who determined the correct identity of OTU A, Dr. Lisa Lumley, Alberta Biodiversity Monitoring Institute, who identified specimens of OTU I, Dr. Dave Walter, University of the Sunshine Coast (Queensland, Australia), who helped with photographic identifications of several mesostigmatans, and Dr. Barry OConnor, University of Michigan (Ann Arbor, Michigan, United States of America), and Dr. Pavel Klimov, Purdue University (West Lafayette, Indiana, United States of America), who provided advice and valuable literature on Astigmata.
Funding statement
This project was supported by a Natural Sciences and Engineering Research Council of Canada (NSERC) Undergraduate Student Research Award (L.-A.B.) and NSERC Discovery Grant (D.P.W.H.).








