Introduction
Diamondback moth, Plutella xylostella (Linnaeus) (Lepidoptera: Plutellidae), is a major pest of economically important Brassicaceae (Zalucki et al. Reference Zalucki, Shabbir, Silva, Adamson, Shu-Sheng and Furlong2012). On a global scale, diamondback moth individuals are genetically similar, with substantial gene flow among distant geographic populations (Caprio and Tabashnik Reference Caprio and Tabashnik1992; Chang et al. Reference Chang, Tabashnik, Artelt, Malvar, Ballester, Ferre and Roderick1997; Li et al. Reference Li, Zhao, Choi, Kim, Sohn and Jin2006; Pichon et al. Reference Pichon, Roux, Kirk, Alauzet, Bordat and Legal2006; Murthy et al. Reference Murthy, Sannaveerappanavar and Shankarappa2014; Perry et al. Reference Perry, Keller and Baxter2020). However, genetically distinct entities (e.g., individuals that resist certain insecticides) may occur within local populations (Tabashnik et al. Reference Tabashnik, Liu, Malvar, Heckel, Masson and Ballester1997). Furthermore, cryptic species may be hidden in populations thought to be diamondback moth (Landry and Hebert Reference Landry and Hebert2013).
In Canada, diamondback moth occurs in all provinces, and widespread (> 1 million ha) outbreaks in canola, Brassica napus Linnaeus and B. rapa Linnaeus (Brassicaceae), have occurred in the Prairie Provinces, resulting in tens of millions of dollars (CAD $) in damage and pest control costs to the canola industry (Dosdall et al. Reference Dosdall, Weiss, Olfert, Mason, Soroka, Shelton, Collins, Zhang and Wu2008; Dosdall and Mason Reference Dosdall, Mason and Williams2010; Isaacs Reference Isaacs2019). It was hypothesised that diamondback moth does not overwinter in Canada (Harcourt Reference Harcourt1963, Reference Harcourt, Talekar and Griggs1986; Butts and McEwen Reference Butts and McEwen1981), although it may in Alberta in some years (Dosdall Reference Dosdall1994) or in southern regions (Dancau et al. Reference Dancau, Mason and Cappuccino2018). Diamondback moth populations from the southern United States of America and northern Mexico are hypothesised to migrate annually on low-level northerly winds into different parts of Canada (Dosdall et al. Reference Dosdall, Mason, Olfert, Kaminski, Keddie, Endersby and Ridland2004; Hopkinson and Soroka Reference Hopkinson and Soroka2010). Diamondback moth populations decline to very low levels or are extirpated in fall due to high mortality caused by abiotic (e.g., rainfall, wind, dislodgement) and biotic factors.
Parasitism can be an important mortality factor of diamondback moth (Shelton et al. Reference Shelton, Wilsey, Hoebeke and Schmaedick2002). A number of parasitoid species have been associated with diamondback moth in Ontario (Harcourt Reference Harcourt1960; Dancau et al. Reference Dancau, Haye, Cappuccino and Mason2020) and the Prairie Provinces (Putnam Reference Putnam1968, Reference Putnam1978; Braun et al. Reference Braun, Olfert, Soroka, Mason, Dosdall, A.A. and Bordat2004; Dosdall and Mason Reference Dosdall, Mason and Williams2010; Bahar et al. Reference Bahar, Soroka, Dosdall and Olfert2013; Fernández-Triana et al. Reference Fernández-Triana, Shaw, Cardinal and Dosdall2014; Munir et al. Reference Munir, Dosdall and O’Donovan2014; Munir Reference Munir2019). However, the parasitoid fauna associated with diamondback moth is unknown or poorly characterised in other parts of Canada. Knowledge of this parasitoid complex could point to vacant niches that might be filled through introductions of effective nonnative species, relocation of parasitoid species within Canada to regions where they do not occur, or the development of strategies to enhance populations of resident species. Furthermore, knowledge of the existing parasitoid fauna can provide guidance for managing diamondback moth under climate change (Furlong and Zalucki Reference Furlong and Zalucki2017).
Life tables are an important tool to understand the factors that contribute to mortality of a pest species and their significance in reducing pest populations. They may also identify niches in life cycles where introductions of natural enemies could be useful. For example, life table studies of the cabbage seedpod weevil, Ceutorhynchus obstrictus Marsham (Coleoptera: Curculionidae), conducted in the area of origin (Haye et al. Reference Haye, Mason, Dosdall and Kuhlmann2010) and area of introduction (Gillespie et al. Reference Gillespie, Broadbent, Mason, Haye, Clarke, Goettel and Leung2019) enabled comparison of the parasitoid fauna between the two areas. Two major parasitoids of C. obstrictus larvae not present in the area of introduction were identified as having high impact in the area of origin. Subsequently, one of these parasitoids was discovered to be already present as an adventive introduction in eastern Canada.
A number of life table studies have been conducted on diamondback moth (e.g., Iga Reference Iga1985; Harcourt Reference Harcourt, Talekar and Griggs1986; Keinmeesuke et al. Reference Keinmeesuke, Vattanatangum, Sarnthoy, Sayampol, Miyata, Saito and Talekar1992; Guilloux et al. Reference Guilloux, Monnerat, Castelo-Branco, Kirk and Bordat2003; Ibohal Singh et al. Reference Ibohal Singh, Jalalp, Rabindra, Rao and Lalitha2004; Golizadeh et al. Reference Golizadeh, Kamali, Fathipour and Abbasipour2009; Peng et al. Reference Peng, Zou, Ren, Xie, Vasseur and Yang2015; Gowri and Manimegalai Reference Gowri and Manimegalai2017; Dancau et al. Reference Dancau, Haye, Cappuccino and Mason2020; Farias et al. Reference Farias, Santos, Carmo, Soares, Costa, Santos and Picanço2021). However, only Iga (Reference Iga1985), Harcourt (Reference Harcourt, Talekar and Griggs1986), Keinmeesuke et al. (Reference Keinmeesuke, Vattanatangum, Sarnthoy, Sayampol, Miyata, Saito and Talekar1992), Dancau et al. (Reference Dancau, Haye, Cappuccino and Mason2020), and Farias et al. (Reference Farias, Santos, Carmo, Soares, Costa, Santos and Picanço2021) examined natural enemies. No study has been done to assess and compare mortality over a broad area. In addition to assessing mortality, life tables can also elucidate natural enemy–feeding niches that could be filled through introduction of biological control agents. For example, Haye et al. (Reference Haye, Dancau, Bennett and Mason2021) conducted a life table study on diamondback moth that elucidated the natural enemy community in Europe, and those results can be compared with results from life table studies in Canada to identify opportunities to fill vacant feeding niches.
The overall objective of the present study was to determine mortality of diamondback moth in different regions of Canada where infestations routinely occur and can be economically important. Specific objectives were: (1) to determine the impact of parasitoids in population dynamics of diamondback moth; (2) to determine the parasitoid species complex and review current knowledge on the main parasitoids associated with diamondback moth; and (3) to assess the potential of introduction of additional parasitoids to enhance mortality due to parasitism.
Materials and methods
Field sites
The study was conducted at Agriculture and Agri-Food Canada (AAFC) Research and Development Centres in four provinces across Canada where diamondback moth populations occur annually. In British Columbia, the experiment was conducted at the AAFC Agassiz Research Farm (49.242662 °N, 121.756807 °W) from 2014 to 2016. The Ontario component was conducted at the Central Experimental Farm, Ottawa (45.388904 °N, 75.722037 °W) from 2016 to 2018. In Prince Edward Island, the experiment was conducted at the Agriculture and Agri-Food Canada Charlottetown (Harrington) Research Farm (46.341542 °N, 63.160312 °W) from 2017 to 2019. The Newfoundland and Labrador component was conducted at the Agriculture and Agri-Food Canada St. John’s Research Farm (47.515941 °N, 52.781857 °W) from 2017 to 2019.
Dancau et al. (Reference Dancau, Haye, Cappuccino and Mason2020) described the major field plot design implemented in each province. Cabbage, Brassica oleracea Linnaeus (Brassicaceae), variety Adaptor, seedlings were planted in May of each year in a tilled 10 m × 10 m plot with individual plants placed every 0.5 m along rows 1 m apart. Within the plot, 20 randomly selected locations were left unplanted to allow placement of sentinel plants infested with the appropriate life stage of diamondback moth. A buffer zone of 44 cabbage plants was planted around the plot, 1 m from the edge, with 1 m between plants to allow detection of incursions from mammalian herbivores – for example, groundhogs, Marmota monax (Linnaeus) (Rodentia: Sciuridae) – before they reached the experimental plants.
Adult monitoring
Two delta-type pheromone traps and diamondback moth lures (Cooper Mills, Madoc, Ontario) were set up at the experimental plot in each province early in the season (14 June 2017 in British Columbia; 11 May 2016, 5 April 2017, and 4 April 2018 in Ontario; 25 April 2017, 16 April 2018, and 8 April 2019 in Prince Edward Island; 19 May 2017, 8 May 2018, and 26 June 2019 in Newfoundland) and removed at the end of the season (24 November 2017 in British Columbia; 23 November 2016, 27 September 2017, and 1 November 2018 in Ontario; 6 October 2017, 25 September 2018, and 16 September 2019 in Prince Edward Island; 5 October 2017, 25 September 2018, and 8 October 2019 in Newfoundland). Traps were equipped with sticky liners that were changed each week, and pheromone lures were replaced every two weeks. Adult diamondback moths were identified visually from sticky cards, and the counts were recorded.
Life table
Destructive sampling of cabbages was attempted to assess mortality of natural diamondback moth populations (see Dancau et al. Reference Dancau, Haye, Cappuccino and Mason2020 for details). Cabbage plants were harvested at soil level 1–3 times during the growing season and carefully examined in the laboratory. The number of diamondback moth eggs, larvae, and pupae was recorded for each plant sampled. The individuals recovered from these samples were maintained in rearing containers under laboratory conditions (approximately 22 ± 1 °C at 60% relative humidity, 16-hour light photoperiod) to assess parasitism levels.
To provide consistency, sentinel plants were infested in the laboratory with known numbers of a single diamondback moth life stage and randomly placed at the vacant locations within each plot (see Dancau et al. Reference Dancau, Haye, Cappuccino and Mason2020 for details). At each location, plants were either caged (control for background mortality) or uncaged (to determine natural enemy mortality) and left in the field until transition to the next life stage began. At this time, plants were removed and processed in the laboratory, where individuals were counted and reared, as described above, to assess parasitism levels and additional mortality.
Diamondback moths used in the sentinel plant experiments were obtained from colonies established from the wild in Agassiz, British Columbia, Ottawa, Ontario (Dancau et al. Reference Dancau, Haye, Cappuccino and Mason2020), and Charlottetown, Prince Edward Island (years 2 and 3). In some years, individuals from the Ottawa colony were sent to Charlottetown (year 1) and St. John’s (all years) to set up temporary colonies for this study.
Discerning generations of diamondback moth using pheromone traps is challenging due to constant immigration. Therefore, as soon as moths were detected in the field, estimates of development using a threshold temperature of 7.3 °C and 293 degree-days for one generation (Butts and McEwen Reference Butts and McEwen1981) were used to determine approximate times for exposing the different life stages of diamondback moth.
Mortality assessment and life table construction
To construct the life tables, mortality rates calculated from field data were applied to a hypothetical cohort of 1000 eggs. Calculation of life table parameters was in accordance with Miall et al. (Reference Miall, Abram, Cappuccino, Bennett, Fernández-Triana, Gibson and Mason2021) and is summarised as follows: apparent mortality (q x ) due to known (e.g., parasitism) or unknown biotic and abiotic factors was calculated as the ratio of the number of individuals dying in a stage (d x ) to the number entering the stage (l x ). Real mortality (r x ) was calculated as the ratio of the number of individuals dying in a particular life stage to the initial starting number at the beginning of the study (1000). To determine the number of individuals entering each life stage, the number dying in each stage was subtracted from the number entering the previous stage. Marginal attack rate (m x ) estimated the number of individuals entering a life stage that would be attacked by an agent (e.g., parasitoids) if it were acting in the absence of other mortality factors. Where overlap between mortality factors occurred or a specific factor was indistinguishable, the marginal attack rate would be designated as equal to the apparent mortality. Once marginal attack rates for each life stage were determined, k-values (level of mortality in a given stage) were calculated as –log (1 − m x ). Each k-value represents the impact of mortality for each life stage on the total generational mortality (K g ), the sum of the k-values. The estimated number of potential progeny was calculated by multiplying the number of surviving females by the potential fecundity (254.88 eggs per female, as used by Dancau et al. Reference Dancau, Haye, Cappuccino and Mason2020). The net reproductive rate of increase (R 0 ) was derived by dividing the estimated number of progeny produced by the initial number of individuals used in the study (1000). The net reproductive rate of increase was defined as the factor by which a population increased (or decreased) in a generation (Van Driesche et al. Reference Van Driesche, Hoddle and Center2009). For sentinel experiments, life tables were generated for each replicate during each year at all locations.
In most life table studies (e.g., Haye et al. Reference Haye, Mason, Dosdall and Kuhlmann2010; Gillespie et al. Reference Gillespie, Broadbent, Mason, Haye, Clarke, Goettel and Leung2019), unknown factors contribute a large proportion of the mortality observed. These may be abiotic (e.g., rainfall, wind, dislodgement) or biotic (e.g., disease and predation by ground dwelling or aerial insects, birds, or small mammals) and are difficult to measure. In this study, rain shelters were set up as a treatment in British Columbia (2015) and Ontario (2016 and 2018). However, the treatment was not expanded to the other provinces and study years because summer rainfall was determined to have a negligible effect on mortality and parasitism.
In the sentinel component of this study, parasitism for each of the egg, larval, and pupal life stages of the host was estimated using the numbers of individuals exposed, the numbers of individuals recovered, and the total numbers of individuals that emerged (parasitoids + adult moths) into the calculation proposed by Dancau et al. (Reference Dancau, Haye, Cappuccino and Mason2020). This calculation included a correction factor for parasitoid-induced mortality:
where N r is the number of recovered individuals, N e is the number of sentinel individuals originally set out, N p is the number of emerged parasitoids, and N m is the number of emerged moths.
In the destructive sampling component of this study, the number of sentinel individuals originally set out was assumed to be the same as the number of recovered individuals (i.e., N e = N r ).
Statistical analyses were not conducted to formally compare parameters among provinces. The data structure allowed only numerical comparisons to be made.
Parasitoids that emerged from field-collected diamondback moth individuals were preserved in 70% ethanol or frozen at –20 °C. Identifications were confirmed by taxonomic experts at the Canadian National Collection of Insects, Arachnids and Nematodes, Ottawa, Ontario, Canada.
Results and discussion
Climate data
Climatic conditions varied among provinces and among years in each province (Table 1). These variations are typical for Canada (Environment and Climate Change Canada 2016). Growing season temperatures were highest in British Columbia and Ontario and coolest in Newfoundland. Although drought conditions were reported during the growing season in all years in British Columbia, rainfall was highest in that province. The other provinces experienced about one-third the rainfall amounts seen in British Columbia, and only Prince Edward Island reported drought conditions in July of each year of the study.
Table 1. Mean (± standard deviation) temperatures (°C) during the growing season (1 May to 30 September), total annual rainfall, and drought conditions at Agassiz, British Columbia, Ottawa, Ontario, Charlottetown, Prince Edward Island, and St. John’s, Newfoundland and Labrador, Canada. Data are from Environment and Climate Change Canada (2020).

Adult populations
Pheromone trap data showed that populations of diamondback moth were highly variable from year to year at all of the study locations (Fig. 1). In Newfoundland, detectable populations of diamondback moth appeared in mid-June (week 24), with noticeable peaks in July and August, before dropping off to zero by mid-September (week 38). Numbers of males captured reached 60 at week 33 in 2019, higher than the peaks recorded in 2017 and 2018 (at weeks 28 and 31, respectively) and the highest number recorded at any location during the study. In Prince Edward Island, detectable diamondback moth populations occurred around mid-May (week 22), remaining below 10 males per week in all years, with peaks in July and August, before declining to zero in mid-September (week 37). In Ontario, diamondback moth populations were first detected in late April or early May (weeks 17–20), with peaks appearing in late May (week 22) and late June into July (weeks 25–28), and dropping off to zero at the end of October (week 45). Numbers of males collected in traps each week were generally below 10, with the exception of week 25 in 2017, when 20 moths were collected. In British Columbia, moth numbers were at their peak (20–25) when pheromone traps were initially set out in 2017 and declined to zero by mid-October (week 43; due to logistical reasons, traps were not set up in 2015 or 2016).
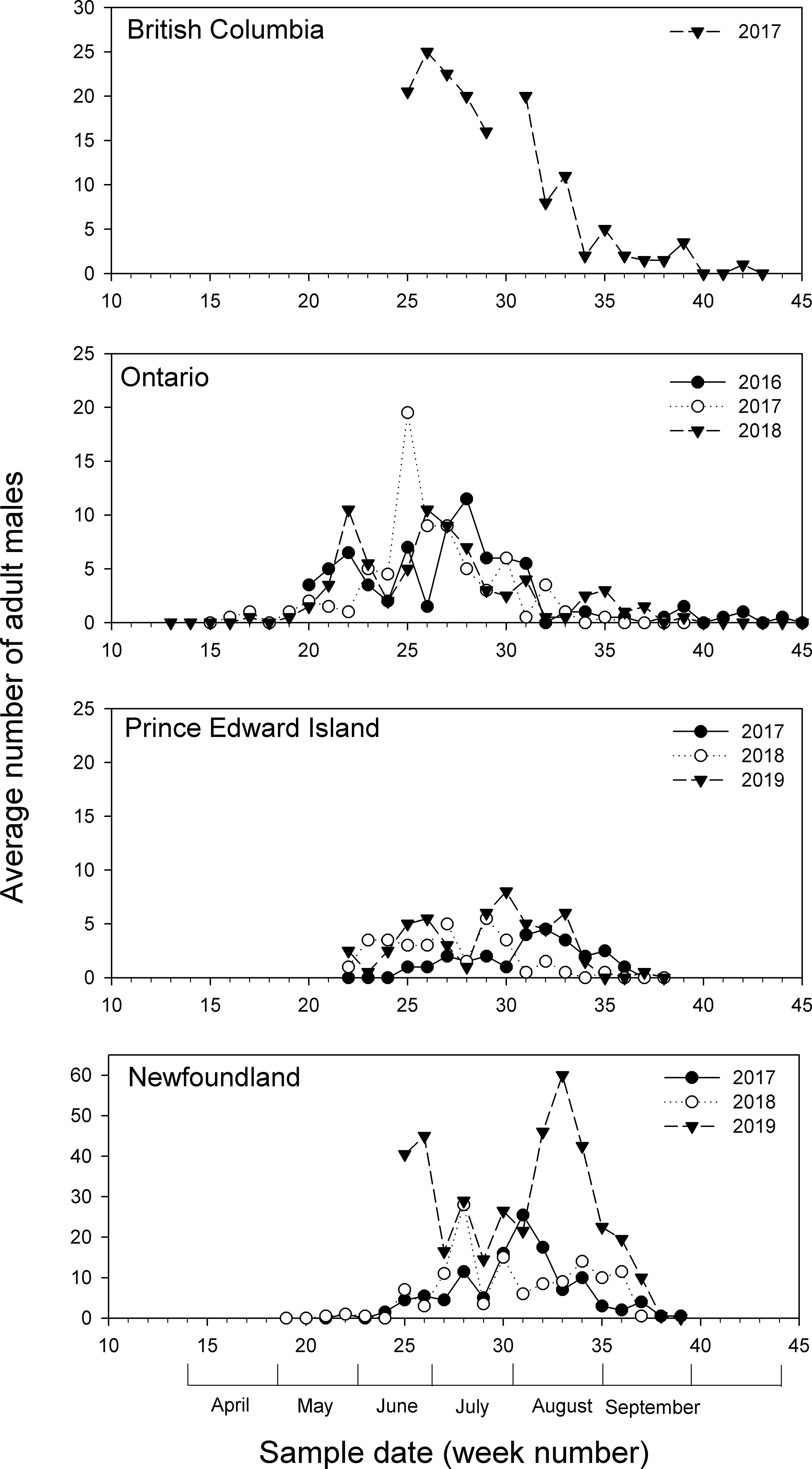
Fig. 1. Average numbers of male Plutella xylostella collected in pheromone traps (n = 2) in British Columbia (2017), Ontario (2016, 2017, 2018), Prince Edward Island (2017, 2018, 2019), and Newfoundland (2017, 2018, 2019) during the growing season.
Although numbers of moths increased and decreased over the field seasons, discrete generations could not be discerned. Since discrete generations could not be discerned, this observation suggests that all life stages could be present in the field at any one time, as was also noted by Harcourt (Reference Harcourt1954).
Life tables
Natural populations of diamondback moth as measured by destructive sampling were highly variable among provinces and years. The numbers of recovered individuals of the egg, larval, and pupal stages combined were very low (data not shown), averaging from zero (Ontario in 2018) to 8.45 ± 1.29 (Prince Edward Island in 2017). Thus, the data collected could not provide the resolution needed for constructing meaningful life tables.
Generational mortality
For sentinel experiments, values generated were highly variable; therefore, data for each location were pooled, and a grand total generational life table was generated for each year (Supplementary material, Tables S1–S25). Total generational mortality of exposed diamondback moth was very high, ranging from 91.8% (Prince Edward Island in 2019) to 99.9% (British Columbia in 2015), compared to that of diamondback moth protected by a control cage, which was highly variable, ranging from 39.2% (Newfoundland in 2017) to 96.1% (Prince Edward Island in 2017; Table 2). Net reproductive rate (R 0 ) was highly variable, with population levels declining (R 0 < 1.0) in most years in British Columbia, Ontario, and Newfoundland. In Prince Edward Island, diamondback moth populations were increasing in each year (R 0 > 1.0) and especially in 2019, when the net reproductive rate reached 10.5 (Table 2). In the two cases where rain shelters were included, generational mortality increased and net reproductive rate decreased only slightly compared to exposed treatments in British Columbia in 2015 – 100 (sheltered) versus 99.9 (exposed); 0.04 (sheltered) versus 0.1 (exposed) – and Ontario in 2018 – 99.8 (sheltered) versus 99.4 (exposed); 0.3 (sheltered) versus 0.7 (exposed). This ‘protection from the elements’ also increased parasitism only slightly – from 5.3% to 6.8% in British Columbia and from 9.7% to 9.8% in Ontario (Table 2).
Table 2. Diamondback moth, Plutella xylostella, net reproductive rate (R 0 ), generational mortality, and mortality due to parasitism for generational life tables at St. John’s, Newfoundland and Labrador (NL), Charlottetown, Prince Edward Island (PE), Ottawa, Ontario (ON), and Agassiz, British Columbia (BC), Canada.
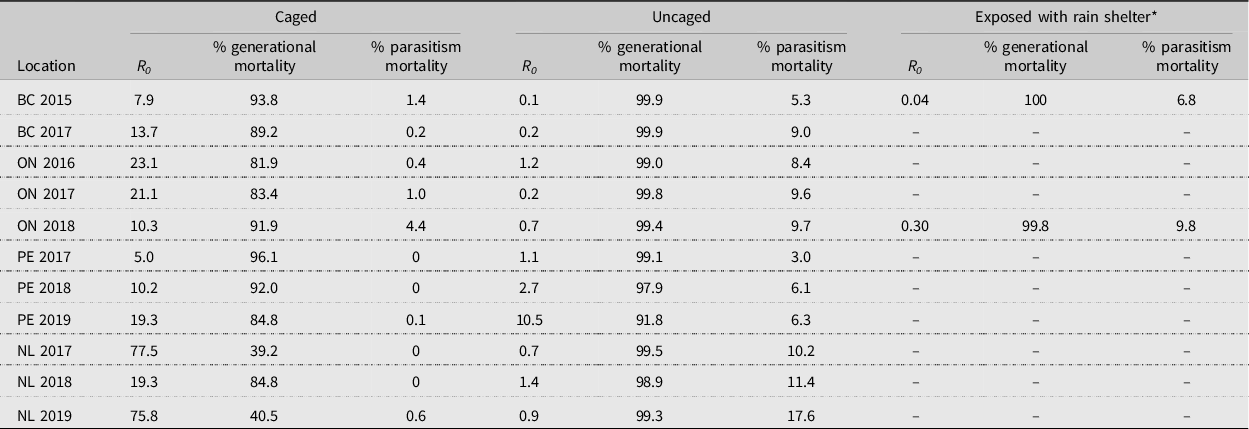
* This treatment was only conducted in British Columbia and Ontario in 2015 and 2018, respectively.
In comparison, Harcourt (Reference Harcourt, Talekar and Griggs1986) and Dancau et al. (Reference Dancau, Haye, Cappuccino and Mason2020) conducted their studies at the same Ontario location as the present study. Furthermore, the 2016 and 2017 Ontario data sets used in the present study are the same as those analysed by Dancau et al. (Reference Dancau, Haye, Cappuccino and Mason2020). Harcourt (Reference Harcourt, Talekar and Griggs1986) developed life tables using a destructive sampling method and found that the generational mortality was 99.5%, resulting in a population decrease. He suggested that a generational mortality of 99.1% would provide for a stable population, whereas a generational mortality of less than 99.1% would result in an increase in the population. The generational mortalities reported in the present study (Table 2) support this hypothesis. Dancau et al. (Reference Dancau, Haye, Cappuccino and Mason2020) reported diamondback moth generational mortalities of 99.96% and 99.97% in 2016 and 2017, respectively. These differ slightly from what is shown in Table 2 (99.0% and 99.8%); the difference is due to a predation correction factor that was used by Dancau et al. (Reference Dancau, Haye, Cappuccino and Mason2020).
Iga (Reference Iga1985) conducted a generational life table study in Japan and determined that apparent mortality of diamondback moth ranged from 88.3% to 99.3% throughout the year. Keinmeesuke et al. (Reference Keinmeesuke, Vattanatangum, Sarnthoy, Sayampol, Miyata, Saito and Talekar1992) conducted a destructive life table study in Thailand and found that mortality of diamondback moth is due to rainfall and parasitism. Overall mortality ranged from approximately 75.5% to 98.3%, with highest values occurring during the wet season, closely followed by the hot season, and lowest mortality occurring during the dry season.
Contribution of parasitism to mortality
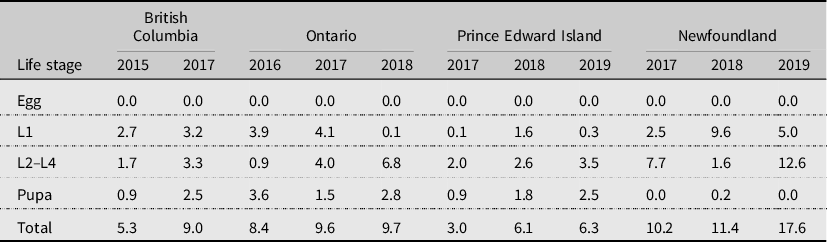
Parasitism has been reported as a significant mortality factor of diamondback moth in many studies (Putnam Reference Putnam1973; Harcourt Reference Harcourt, Talekar and Griggs1986; Lasota and Kok Reference Lasota and Kok1986; Fox et al. Reference Fox, Letourneau, Eisenbaeh and Van Nouhuys1990; Muckenfuss et al. Reference Muckenfuss, Sheppard, Ferrer and Talekar1992; Wakisaka et al. Reference Wakisaka, Tsukuda, Nakasuji and Talekar1992; Haseeb et al. Reference Haseeb, Kobori, Amano and Nemoto2001; Xu et al. Reference Xu, Shelton and Cheng2001; Shelton et al. Reference Shelton, Wilsey, Hoebeke and Schmaedick2002; Bahar et al. Reference Bahar, Soroka, Dosdall and Olfert2013; Qureshi et al. Reference Qureshi, ul Mohsin, Naeem and Shah2020). In particular, the larval–prepupal parasitoid Diadegma insulare (Cresson) (Hymenoptera: Ichneumonidae) has been reported to be responsible for up to 50–95% parasitism (e.g., Lasota and Kok Reference Lasota and Kok1986; Xu et al. Reference Xu, Shelton and Cheng2001; Shelton et al. Reference Shelton, Wilsey, Hoebeke and Schmaedick2002). In the present study, parasitism was generally low and varied among provinces and among years (Table 3). Overall, parasitism contributed from 3.0% to 17.6% to mortality of diamondback moth (Table 2). It was highest in Newfoundland (from 10.2% to 17.6%), moderate in Ontario (from 8.4% to 9.7%), lowest in Prince Edward Island (from 3.0% to 6.3%), and British Columbia had both low (5.3% in 2015) and moderate (9.0% in 2017) parasitism levels (Table 2). Parasitism varied among life stages, with parasitism of diamondback moth eggs being 0%, parasitism of first-instar larvae ranging from 0.1% (Prince Edward Island in 2017 and Ontario in 2018) to 9.6% (Newfoundland in 2018), parasitism of second- to fourth-instar larvae ranging from 0.9% (Ontario in 2016) to 12.6% (Newfoundland in 2019), and parasitism of pupae ranging from 0% (Newfoundland in 2017) to 3.6% (Ontario in 2016; Table 3). The highly variable results may be due to experimental design (e.g., sentinel individuals versus individuals from natural populations), placement of infested sentinel plants asynchronous with the natural presence of parasitoids, landscape effects, or abiotic factors. For example, mortality due to parasitism in British Columbia was 5.3% in 2015 versus 10.8% in 2017. This may have been influenced by the significant prolonged drought conditions in 2015 (May to September, with extreme conditions in July) compared to less-severe dry conditions in 2017 (July and August) in British Columbia. Lower mortality due to parasitism in Prince Edward Island, compared to that at all other locations, may partially explain the increasing diamondback moth populations in all years in that province; however, other mortality factors may also have had less impact.
Table 3. Generational mortality (%) contributed by parasitism to reduction of each life stage of diamondback moth, Plutella xylostella, at Agassiz, British Columbia, Ottawa, Ontario, Charlottetown, Prince Edward Island, and St. John’s, Newfoundland and Labrador, Canada.
Abiotic factors
Among abiotic factors, high mortalities have been associated with thunderstorm activity and rainfall during periods of cool weather (Harcourt Reference Harcourt1963), but rainfall patterns are highly variable during the growing season and among years. Treatments that excluded rainfall were conducted in British Columbia (2015) and Ontario (2016 and 2018). The data show that overall mortality of diamondback moth on sentinel plants exposed to the elements differed little from that on plants protected from rain, although mortality due to parasitism increased slightly (Table 2). However, in 2015, British Columbia experienced moderate-to-severe drought conditions in most months of the study, and conditions were extreme in July (Table 1). In contrast, although average rainfall occurred at the Ottawa location in 2018, generational mortality differed by only 0.4%. As was also reported by Dancau et al. (Reference Dancau, Haye, Cappuccino and Mason2020), severe drought conditions occurred at the Ottawa location in June and July 2016, and rain shelter treatments had no effect on generational mortality. Other studies have found that rainfall can have a significant impact on mortality of diamondback moth. For example, a life table study in Japan showed that 38% of mortality resulted from eggs being washed off by rain (Sivapragasam Reference Sivapragasam1986, as cited in Keinmeesuke et al. Reference Keinmeesuke, Vattanatangum, Sarnthoy, Sayampol, Miyata, Saito and Talekar1992). The study by Iga (Reference Iga1985) showed that diamondback mortality was highest during the wet season and lowest during the dry season. Farias et al. (Reference Farias, Santos, Carmo, Soares, Costa, Santos and Picanço2021) found that rainfall caused 44.5% mortality of diamondback moth in a study in Brazil.
Other biotic factors
Mortality of diamondback moth is also due to several biotic factors, such as pathogens – which can be challenging to determine – and predators. Although not a focus of the present study, predation can be measured indirectly by comparing mortality between caged and uncaged treatments (Table 2) and by documenting the fauna present at a study site. Dancau et al. (Reference Dancau, Haye, Cappuccino and Mason2020) found that predation accounted for a substantial proportion of diamondback moth mortality at the Ottawa study site in 2017. Other studies have shown that invertebrate predators can be a significant mortality factor of diamondback moth (Muckenfuss et al. Reference Muckenfuss, Sheppard, Ferrer and Talekar1992; Farias et al. Reference Farias, Santos, Carmo, Soares, Costa, Santos and Picanço2021).
Parasitoid complex
The parasitoid complex associated with diamondback moth in this study consisted of 10 species in the families Hymenoptera, Braconidae, Chalcididae, Eulophidae, Ichneumonidae, and Pteromalidae (Tables 4 and 5). Among these, Microplitis plutellae Muesebeck (Hymenoptera: Braconidae), Diadegma insulare, and Diadromus subtilicornis (Gravenhorst) (Hymenoptera: Ichneumonidae) were the most common species found across Canada. It is noteworthy that, although increasing numbers of adventive parasitoid species are being recorded in North America (Weber et al. Reference Weber, Hajek, Hoelmer, Schaffner, Mason, Stouthamer and Mason2021), the widespread and highly successful diamondback moth pupal parasitoid Diadromus collaris (Gravenhorst) (Hymenoptera: Ichneumonidae) was not recovered during this study (see Haye et al. Reference Haye, Dancau, Bennett and Mason2021).
Table 4. Cumulative percent parasitism (n =) by parasitoids (Hymenoptera) in sentinel-based life tables for diamondback moth, Plutella xylostella, based on uncaged sentinel plants in cabbage plots at Agassiz, British Columbia, Ottawa, Ontario, Charlottetown, Prince Edward Island and St. John’s, Newfoundland and Labrador, Canada.
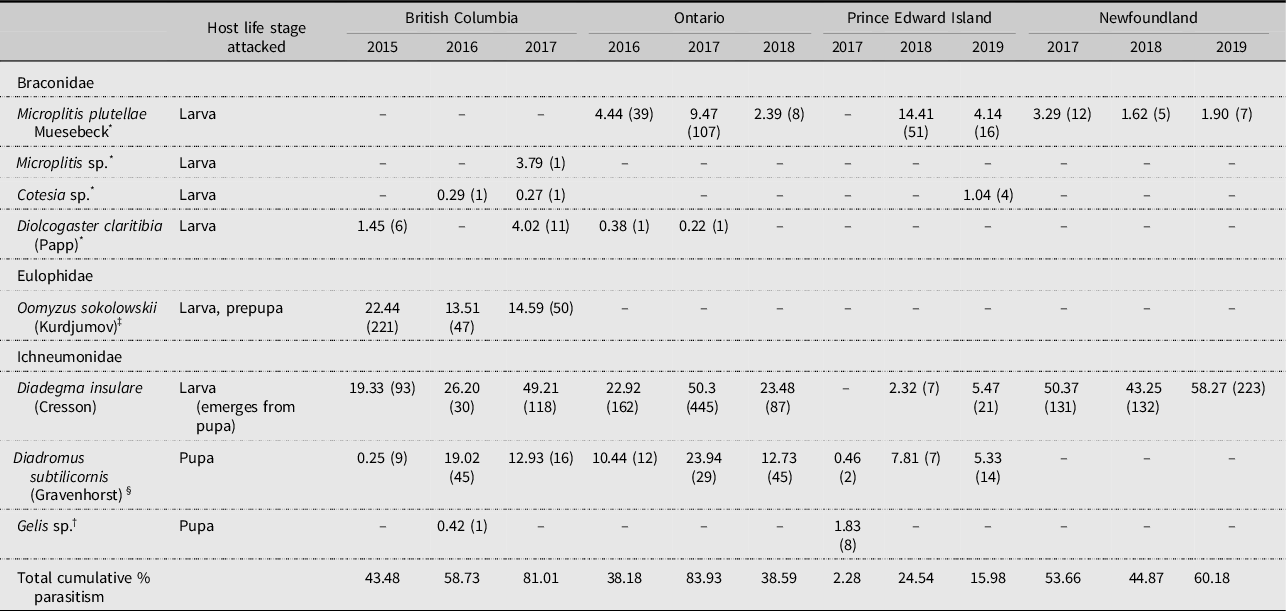
* primary parasitoid;
† facultative hyperparasitoid;
‡ LaSalle (Reference LaSalle1994) stated that Oomyzus sokolowskii can be a facultative hyperparasitoid, and Graham (Reference Graham1991, p. 204) mentioned that it also attacks the primary parasitoid, Cotesia vestalis (mentioned in that reference as Apanteles plutellae Kurdjumov (Hymenoptera: Braconidae)) in Europe. However, there are no reports of O. sokolowskii hyperparasitism in North America (J.T. Huber, personal communication).
Table 5. Cumulative percent parasitism (n =) by parasitoids (Hymenoptera) in destructive sampling-based life tables for diamondback moth, Plutella xylostella, in cabbage plots at Agassiz, British Columbia, Ottawa, Ontario, Charlottetown, Prince Edward Island, and St. John’s, Newfoundland and Labrador, Canada.

Levels of parasitism differed among years and locations for each of the life stages (Supplementary material, Tables S26–S29). Parasitoids were present in all years at all locations (Tables 4 and 5): D. insulare was the most frequently present, followed by D. subtilicornis and M. plutellae. Differences among provinces were evident: for example, M. plutellae was present in all provinces except British Columbia, apparently being replaced there by another Microplitis sp. and O. sokolowskii, the latter species occurring elsewhere only in Ontario in one study year (2016). Of note is the presence of the generalist facultative hyperparasitoids Conura albifrons (Walsh) (Hymenoptera Chalcididae) and Gelis sp. (Hymenoptera: Ichneumonidae) and the obligate hyperparasitoids Pteromalus sp. and Trichomalopsis dubia (Ashmead) (both Hymenoptera: Pteromalidae), the latter two recovered only from natural diamondback moth populations (Table 5).
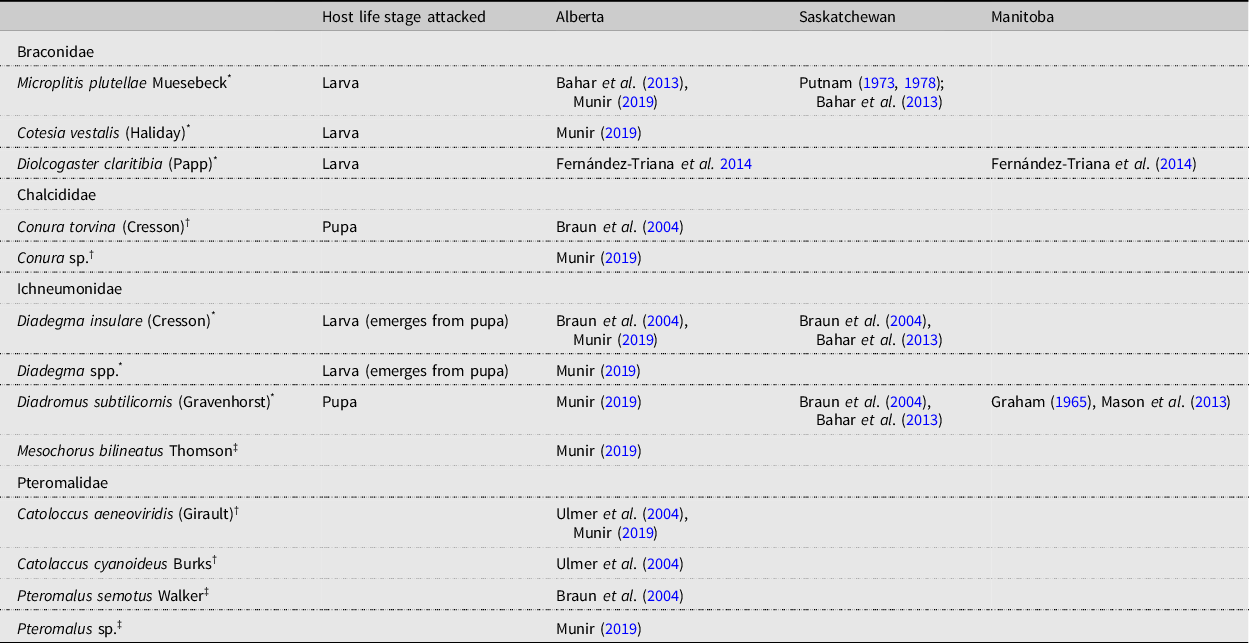
The parasitoid complex associated with diamondback moth populations documented in the present study is similar to that found on the Canadian prairies (Table 6), with M. plutellae, D. insulare, and D. subtilicornis being the main species reported. Of interest is the presence of several Pteromalidae species in Alberta.
Table 6. Parasitoids associated with diamondback moth, Plutella xylostella, in Alberta and Saskatchewan, Canada.
* primary parasitoid;
† facultative hyperparasitoid;
‡ obligate hyperparasitoid.
Diamondback moth has been presumed not to overwinter in most of Canada (Harcourt Reference Harcourt1960; Butts and McEwen Reference Butts and McEwen1981; Dancau et al. Reference Dancau, Mason and Cappuccino2018) and is hypothesised to migrate from southern locations (Hopkinson and Soroka Reference Hopkinson and Soroka2010). It has also been assumed that the major parasitoids D. insulare and D. subtilicornis also migrate. However, this has never been supported by evidence, and the finding that M. plutellae overwinters in Saskatchewan (Putnam Reference Putnam1978) suggests that some parasitoids do not migrate. Review of the distribution, host range, and biology of the other two major parasitoid species could provide clues as to whether they migrate.
Current knowledge of the main parasitoids found across Canada
Diadegma insulare . One of the most common parasitoids of diamondback moth, D. insulare, is primarily a New World species ranging from Canada to Argentina (Azidah et al. Reference Azidah, Fitton and Quicke2000). In North America, D. insulare has been reported from Mexico, at least 15 states in the continental United States of America, and in all Canadian provinces from Québec westward, as well as being newly reported in Prince Edward Island (Noronha and Bahar Reference Noronha and Bahar2018), New Brunswick (Bennett, unpublished data), and Newfoundland and Labrador (Parsons and Dixon, unpublished data).
Five host species, including diamondback moth, have been associated with D. insulare (Yu et al. Reference Yu, van Achterberg and Horstmann2016; Sourakov and Mitchell Reference Sourakov and Mitchell2020). Among these, Phthorimaea operculella (Zeller) (Lepidoptera: Gelechiidae), the potato tuber moth and Plutella armoraciae Busck (Lepidoptera: Plutellidae) occur in Canada. Phthorimaea operculella is resident in British Columbia, Manitoba, and Ontario, and within human environments in Québec (Pohl et al. Reference Pohl, Landry, Schmidt, Lafontaine, Troubridge and MaCauley2018). However, the association of D. insulare with P. operculella is based on a single record (Izhevskiy Reference Izhevskiy1985, as cited in Yu et al. Reference Yu, van Achterberg and Horstmann2016) and may be a misidentification of Diadegma pulchripes (Kokujev) (Hymenoptera: Ichneumonidae), which is a known associate in the Palaearctic region (Coll et al. Reference Coll, Gavish and Dori2000; Pucci et al. Reference Pucci, Spanedda and Minutoli2003; Rondon Reference Rondon2010). Although Idris and Grafius (Reference Idris and Grafius2001) successfully reared D. insulare from P. operculella in the laboratory, they did not rear this parasitoid from field-collected P. operculella hosts. Diadegma insulare has been reared from Plutella armoraciae in Colorado (Marsh Reference Marsh1913). This host species is also native to British Columbia and is found coexisting with diamondback moth in semi-arid habitats in the province’s interior (Abram et al. unpublished data). Diadegma insulare has also been reared from Plutella omissa Walsingham (Marsh Reference Marsh1913). There are an additional four Plutella spp. found in Canada, of which three are native: Plutella haasi Staudinger (Alberta, Québec, Labrador), Plutella notabilis Busck (Yukon, Alberta), and Plutella polaris Zeller (Alaska, Northwest Territories, Nunavut, Québec), and the introduced Plutella porrectella (Linnaeus) (British Columbia, Alberta, Ontario, Québec, Newfoundland). All of these species may be hosts for D. insulare, and they may serve as overwintering reservoirs, providing an annual seed population of D. insulare. However, D. insulare has not been associated with P. porrectella (Smith and Sears Reference Smith and Sears1984). The parasitoid associates of P. haasi and the northern species, P. notabilis and P. polaris, are also unknown. Hellula undalis (Fabricius) (Lepidoptera: Crambidae), the cabbage or Old World webworm, was reported by Sourakov and Mitchell (Reference Sourakov and Mitchell2020) as a host of D. insulare. However, this may be a misidentification of the cabbageworm Hellula rogatalis (Hulst) (Lepidoptera: Crambidae) (Schmidt, personal communication), which sporadically occurs in eastern provinces and may be resident in southern parts of Québec and Ontario (Pohl et al. Reference Pohl, Landry, Schmidt, Lafontaine, Troubridge and MaCauley2018).
The biology of D. insulare is well known and was reviewed by Sarfraz et al. (Reference Sarfraz, Keddie and Dosdall2005) and Munir et al. (Reference Munir, Dosdall and O’Donovan2014). Its life cycle is well synchronised with diamondback moth (Bolter and Laing Reference Bolter and Laing1983), and in North America, D. insulare can parasitise 50–70% of larval hosts (first- to fourth-instar larvae; Lasota and Kok Reference Lasota and Kok1986; Xu et al. Reference Xu, Shelton and Cheng2001) and in some situations up to 95% of larval hosts (Muckenfuss et al. Reference Muckenfuss, Sheppard, Ferrer and Talekar1992). Among the factors allowing D. insulare to be highly successfully are the close spatial associations between densities of this larval parasitoid and P. xylostella, indicating that host abundance is a main determinant of parasitoid distribution patterns (Sarfraz et al. Reference Sarfraz, Dosdall, Black and Keddie2010). In southern climates, it is reported that D. insulare overwinters in the cocoon (Sourakov and Mitchell Reference Sourakov and Mitchell2020). It has been widely assumed that D. insulare migrates into Canada with diamondback moth; however, no conclusive proof has been provided. Although Putnam (Reference Putnam1968) conducted a field cage experiment and found no evidence that D. insulare entered diapause or that it could survive winter conditions in Saskatchewan, a more recent study found that D. insulare overwintered in larvae of P. armoraciae (Abram et al. unpublished data).
Microplitis plutellae . This species is widespread in the Holarctic region (Yu et al. Reference Yu, van Achterberg and Horstmann2016; Fernández-Triana et al. Reference Fernández-Triana, Shaw, Boudreault, Beaudin and Broad2020). In North America, it has been reported from Alberta, Saskatchewan, Ontario, and Québec and is newly reported in Prince Edward Island (Noronha and Bahar Reference Noronha and Bahar2018), Newfoundland (the present study) and Labrador (Parsons and Dixon, unpublished data), and in at least 12 states in the United States of America. Recently, M. plutellae has also been reported from British Columbia, Manitoba, and New Brunswick (Fernández-Triana, unpublished data).
Two host species in addition to diamondback moth have been associated with M. plutellae (Oatman Reference Oatman1966; Yu et al. Reference Yu, van Achterberg and Horstmann2016). Microplitis plutellae was reared from cabbage looper, Trichoplusia ni (Hübner) (Lepidoptera: Noctuidae) (Oatman Reference Oatman1966; Oatman and Platner Reference Oatman and Platner1969; Oatman et al. Reference Oatman, Platner, Wyman, Steenwyk, van Johnson and Browning1983) and from the imported cabbageworm, Pieris rapae (Linnaeus) (Lepidoptera: Pieridae) (Oatman Reference Oatman1966; Oatman and Platner Reference Oatman and Platner1969) in southern California. Other studies of the parasitoids of T. ni have not documented M. plutellae as a parasitoid of this host. For example, Murillo et al. (Reference Murillo, Hunt and Vanlaerhoven2012) reared Microplitis alaskensis Ashmead (Hymenoptera: Braconidae) among nine primary parasitoids from T. ni in southwestern Ontario but not M. plutellae. Pieris rapae is present in all Canadian provinces and territories except the Yukon (Pohl et al. Reference Pohl, Landry, Schmidt, Lafontaine, Troubridge and MaCauley2018). Microplitis plutellae is not listed among the 50 parasitoid species associated with P. rapae in the Centre for Agriculture and Bioscience International Invasive Species (2020) Compendium.
The biology of M. plutellae, a larval parasitoid of diamondback moth, is well known and was reviewed by Bolter and Laing (Reference Bolter and Laing1983), Sarfraz et al. (Reference Sarfraz, Keddie and Dosdall2005), and Munir et al. (Reference Munir, Dosdall and O’Donovan2014). Harcourt (Reference Harcourt1963), Putnam (Reference Putnam1968, Reference Putnam1973), and Bolter and Laing (Reference Bolter and Laing1983) described the interactions between M. plutellae and D. insulare and concluded that M. plutellae was an important early-season parasitoid, but its numbers declined in subsequent generations. In a 10-year study in Saskatchewan, parasitism by M. plutellae averaged 51% (24–77%) of first-generation diamondback moth larvae and 16% (5–33%) of second-generation larvae (Putnam Reference Putnam1973). Subsequent studies have confirmed this dynamic (e.g., Xu et al. Reference Xu, Shelton and Cheng2001). Putnam (Reference Putnam1968) determined that a portion of each M. plutellae generation entered diapause and overwintered in Saskatchewan, with a portion of the population even surviving for two winters. He suggested that this would provide a reservoir population to compensate for years when diamondback moth hosts were not present.
Diadromus subtilicornis . This pupal parasitoid is widespread in the Holarctic region, having been reported from more than 30 countries (Yu et al. Reference Yu, van Achterberg and Horstmann2016). In North America, D. subtilicornis has been reported from British Columbia, Alberta, Saskatchewan, Manitoba, Ontario, Québec, and five mid-western and eastern states in the United States of America (Braun et al. Reference Braun, Olfert, Soroka, Mason, Dosdall, A.A. and Bordat2004; Yu et al. Reference Yu, van Achterberg and Horstmann2016; Munir Reference Munir2019), and it is newly reported in Prince Edward Island (Noronha and Bahar Reference Noronha and Bahar2018) and Newfoundland and Labrador (Parsons and Dixon, unpublished data).
In addition to diamondback moth, D. subtilicornis has been associated with seven other host species (Yu et al. Reference Yu, van Achterberg and Horstmann2016). Among these, only Plutella porrectella (Linnaeus) (Lepidoptera: Plutellidae) and Acrolepiopsis assectella Zeller (Lepidoptera: Glyphipterigidae) occur in Canada. Although Diller and Shaw (Reference Diller and Shaw2014) reported D. subtilicornis from A. assectella in the United Kingdom, extensive rearing of parasitoids from field-exposed A. assectella in Ontario has yielded no D. subtilicornis despite the abundance of individuals of this parasitoid being reared from diamondback moth (Mason et al. Reference Mason, Brauner, Miall and Bennett2013). A single D. subtilicornis was reared from P. porrectella in British Columbia (Mason et al. Reference Mason, Brauner, Miall and Bennett2013); despite this host being widely distributed in British Columbia, Alberta, Ontario, Québec, and Newfoundland (Pohl et al. Reference Pohl, Landry, Schmidt, Lafontaine, Troubridge and MaCauley2018), no other parasitoid records have been documented (e.g., Smith and Sears Reference Smith and Sears1984). Among the other five hosts associated with D. subtilicornis are two Plutellidae species, Eidophasia messingiella (Fischer von Röslerstamm) and Rhigognostis senilella (Zetterstedt), which are European (Diller and Shaw Reference Diller and Shaw2014). In addition, there is one non-Nearctic Glyphipterigidae species that is a host of D. subtilicornis: Acrolepia alliella (Semenov and Kuznetzov) in Japan (Kusigemati Reference Kusigemati1981). Given the fact that there is another substantiated host record from a glyphipterigid, this association seems credible.
The biology of D. subtilicornis, a prepupal and pupal parasitoid of diamondback moth, is relatively well known. Overall, parasitism of diamondback moth by D. subtilicornis is relatively low, ranging from 0.4% to 16% (Harcourt Reference Harcourt1960; Shelton et al. Reference Shelton, Wilsey, Hoebeke and Schmaedick2002; Braun et al. Reference Braun, Olfert, Soroka, Mason, Dosdall, A.A. and Bordat2004; Dosdall et al. Reference Dosdall, Mason, Olfert, Kaminski, Keddie, Endersby and Ridland2004). Females discriminate between younger hosts used for oviposition (prepupae and 1- to 2-day-old green pupae) and older hosts (1- to 3-day-old beige pupae) used for host feeding (Tran and Takasu Reference Tran and Takasu2000).
Oomyzus sokolowskii . This larval–pupal parasitoid has a global distribution (Noyes Reference Noyes2019). In North America, it has been reported from Ontario, Florida, Missouri, and New York (Noyes Reference Noyes2019), and it is newly reported in British Columbia (current study) and Newfoundland (Parsons and Dixon, unpublished data).
In addition to diamondback moth, O. sokolowskii has been associated with Pieris brassicae Linnaeus (Lepidoptera: Pieridae) and an unidentified Plutella sp. (Noyes Reference Noyes2019). Pieris rapae, a sister species of P. brassicae, is a northern temperate species with origins in Europe and was accidentally introduced into North America in the 1860s. However, O. sokolowskii is not listed among the 50 known parasitoids associated with P. rapae (Centre for Agriculture and Bioscience International 2020). As noted under D. insulare, there are five Plutella spp. in addition to diamondback moth that are resident in Canada (Pohl et al. Reference Pohl, Landry, Schmidt, Lafontaine, Troubridge and MaCauley2018). Plutella armoraciae is native and coexists with diamondback moth in grassland habitats in British Columbia (Abram et al., unpublished data). Oomyzus sokolowskii has not been associated with Plutella porrectella, a European immigrant.
Oomyzus sokolowskii parasitises second- to fourth-instar larvae and prepupae of diamondback moth (Talekar and Hu Reference Talekar and Hu1996), with a preference for fourth-instar larvae (Sow et al. Reference Sow, Arvanitakis, Niassy, Diarra and Bordat2013). Parasitism levels in North America are variable (Harcourt Reference Harcourt1960; Shelton et al. Reference Shelton, Wilsey, Hoebeke and Schmaedick2002). LaSalle (Reference LaSalle1994) stated that O. sokolowskii can be a facultative hyperparasitoid, and Graham (Reference Graham1991, p. 204) mentioned that it also attacks the primary parasitoid, Cotesia vestalis (Haliday) (mentioned in that reference as Apanteles plutellae Kurdjumov) (Hymenoptera: Braconidae) in Europe. However, there are no reports of O. sokolowskii hyperparasitism in North America (J.T. Huber, personal communication).
Diolcogaster claritibia . This larval parasitoid is widespread in the Palaearctic region, but in North America, it is found only in Canada (Fernández-Triana et al. Reference Fernández-Triana, Shaw, Cardinal and Dosdall2014, Reference Fernández-Triana, Shaw, Boudreault, Beaudin and Broad2020; Yu et al. Reference Yu, van Achterberg and Horstmann2016). It was initially reported from Alberta, Manitoba, and Ontario, and it was considered a likely recent introduction from Europe to North America (Fernández-Triana et al. Reference Fernández-Triana, Shaw, Cardinal and Dosdall2014). Here, we also report it from British Columbia, Québec, and Saskatchewan (based on specimens sent to the National Identification Service of the Canadian National Collection of Insects, Arachnids and Nematodes and examined by JFT for this study). Diolcogaster claritibia has been associated only with diamondback moth (Yu et al. Reference Yu, van Achterberg and Horstmann2016), but otherwise the biology of this species is unknown. As a new arrival in Canada, D. claritibia may yet become an important parasitoid of diamondback moth because new reports from across Canada are increasing. Ongoing monitoring for this species will provide the data to verify this.
Potential for introduction of additional parasitoids
In other regions of the world, introductions of Cotesia vestalis, Oomyzus sokolowskii, Diadegma semiclausum Hellén (Hymenoptera: Ichneumonidae), and Diadromus collaris have enhanced suppression of diamondback moth by native parasitoids, with the latter two species being the most successful (Sarfraz et al. Reference Sarfraz, Keddie and Dosdall2005 and references therein). Although C. vestalis and O. sokolowskii are present in Canada, D. semiclausum and D. collaris are not present in North America. Consideration should therefore be given to the potential for introduction of one or both species to enhance suppression of diamondback moth populations in some or all regions of Canada. For example, would introduction of the pupal parasitoid D. collaris increase parasitism mortality in Prince Edward Island or Newfoundland and Labrador where D. subtilicornis has little or no impact? In addition, would introduction of D. semiclausum, D. collaris, or another species improve parasitism mortality in Ontario and British Columbia?
Life table studies in Europe have shown that cumulative parasitism by Diadegma fenestrale (Holmgren) (Hymenoptera: Ichneumonidae) and D. semiclausum as high as 48% (Haye et al. Reference Haye, Dancau, Bennett and Mason2021), suggesting that targeting increases in larval parasitism with potential parasitoid introductions into Canada could be achievable. Exposure of sentinel pupae in Swiss canola fields showed that, in warm dry years, pupal parasitism can average 30% (Haye, unpublished data). Haye et al. (Reference Haye, Dancau, Bennett and Mason2021) also found that the larval–pupal parasitoids D. semiclausum and D. fenestrale provided high levels of parasitism throughout the season, with D. fenestrale being more abundant early in the season and D. semiclausum later in the season. The introduction of D. fenestrale could increase the early season mortality of diamondback moth populations in Canada and thus reduce population densities later in the season.
Understanding of the potential role of increased pupal and larval parasitism of diamondback moth in Canada can be gained by examining parasitism levels of parasitoids attacking these two life stages in the context of how mortality is structured among life stages, as estimated by our life table studies. We examined this by averaging apparent mortality values for each life stage across years and for each location (using only years for which the life table methodology was consistent) and then varying the proportion of pupal and larval parasitism above and below observed values to determine the response of estimated diamondback moth net reproductive rate (R 0 ; Fig. 2).
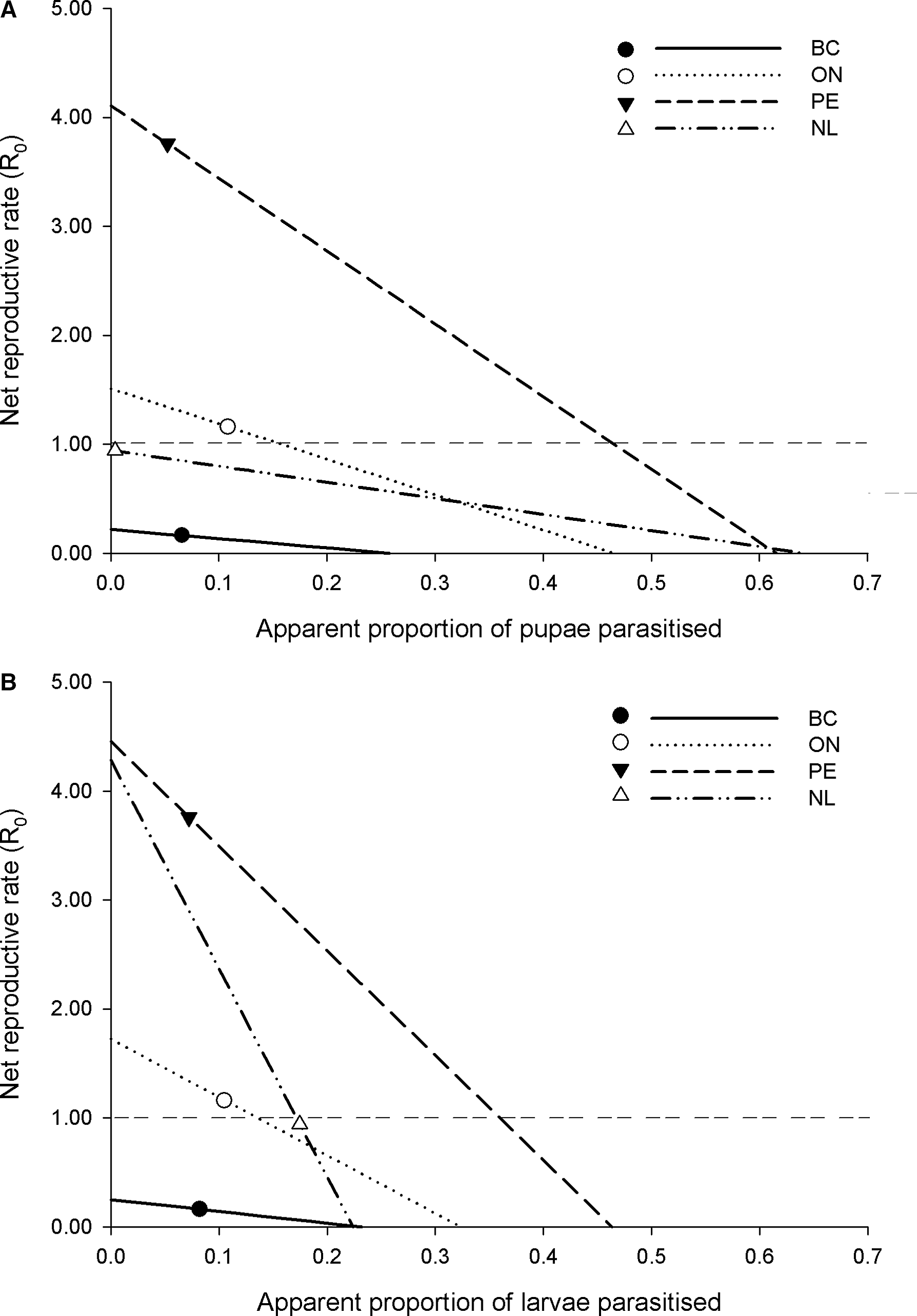
Fig. 2. The theoretical response of the net reproductive rate (R 0 ) of Plutella xylostella populations to A, pupal and B, larval parasitism, based on among-year averages of observed stage-specific apparent mortality levels of exposed individuals in British Columbia (BC), Ontario (ON), Prince Edward Island (PE), and Newfoundland and Labrador (NL) (in each panel, all other parameters are held constant for varying levels of pupal and larval parasitism). The symbols on each line show the observed average proportion of pupae (A) or larvae (B) parasitised and the corresponding estimate of R 0 . The horizontal dashed line indicates an R 0 = 1.0, that is, the threshold between a growing and declining population.
The main conclusion gained from this analysis is that the R 0 values of Canadian diamondback moth populations are predicted to respond more to increases in larval parasitism than to increases in pupal parasitism (Fig. 2). For example, in Prince Edward Island and Ontario, which on average are estimated to have growing within-season diamondback moth populations, 41% and 5% increases in apparent pupal parasitism would be required, respectively, to reduce R 0 below 1.0 (Fig. 2A). It would be predicted to take considerably smaller increases in larval parasitism – 29% and 3% increases for Prince Edward Island and Ontario, respectively – to reduce diamondback moth R 0 below 1.0 (Fig. 2B). For locations with declining within-season diamondback moth populations (Newfoundland and British Columbia), pupal parasitism does not factor into growing or declining populations: even reducing pupal parasitism to zero would not result in a growing diamondback moth population. For Newfoundland, however, even modestly lower levels of larval parasitism (i.e., approximately a 2% decrease) would be predicted to bring R 0 above 1.0. The specific values of parasitism cited above would obviously be sensitive to year-over-year variation in mortality factors and other demographic parameters (e.g., fecundity of diamondback moth). However, the greater efficacy of parasitoids attacking earlier life stages of pests, when other mortality factors act throughout the life cycle, is a well-known phenomenon predicted by biological control theory and supported by empirical evidence (Murdoch and Briggs Reference Murdoch and Briggs1996) and would be predicted to be a general phenomenon in the diamondback moth system.
Conclusions
Generational mortality of diamondback moth is substantial in all regions of Canada where it occurs. In the present study, the net reproductive rate of population increase was generally less than 1.0, suggesting that populations declined over the season, except in Prince Edward Island where populations increased in all years. A very large part of this mortality (> 81%) was due to a variety of factors, many of which cannot easily be identified (e.g., abiotic factors, disease, predation). One that can be identified is parasitism, which contributed from 3.0% to 17.6% of the mortality observed but was very low compared to mortality by parasitism in other regions, as documented in Europe by Haye et al. (Reference Haye, Dancau, Bennett and Mason2021). However, as Dancau et al. (Reference Dancau, Haye, Cappuccino and Mason2020) and Hopkinson and Soroka (Reference Hopkinson and Soroka2010) reported, low-level winds from southern North America are associated with appearance of diamondback moth populations, and it is plausible that these events bring new immigrants to the study locations frequently, thereby effectively compensating for population decreases.
The findings presented here highlight the limitations of using a sentinel-type life table approach. Because this experimental design requires that high densities of hosts be used to quantify mortality, these densities may attract only the most common parasitoid species present in the system. As noted when comparing the parasitoid complexes from sentinel samples (Table 4) with those from destructive (natural population) samples (Table 5), the natural population yielded a greater diversity.
Three species – the widespread Diadegma insulare, Microplitis plutellae, and Diadromus subtilicornis – are the main parasitoids impacting diamondback moth in Canada. Our study suggests that regional differences exist. Overwintering of parasitoids is possible where conditions are suitable and alternate hosts are present. Further study of overwintering and alternate host use by the key parasitoids of diamondback moth will contribute to better understanding of host–parasitoid interactions and the potential for additional species to be introduced for biological control.
The introduction of one or more parasitoids from Europe (e.g., Diadegma semiclausum, Diadegma fenestrale, or Diadromus collaris) could enhance biological control of the existing parasitoid complex. Future life table analyses would be needed to understand how increasing larval and pupal parasitism levels would interact to reduce diamondback moth population growth rates across regions and how that dynamic could play out between migrant and resident diamondback moth populations. However, further studies are needed to elucidate the potential impact and safety of introducing one or more of these candidate biological control agents.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.4039/tce.2021.51.
Acknowledgements
The authors would like to thank Ana Maria Farmakis, Andrew Chaulk, Christine Cock, Ashley Davies-Marsh, Nadine Gaskell, Jessica Leung, Brendan Meaney, Todd Power, Alicia Rochette, and Margie Wilkes for technical support. Gary A.P. Gibson identified the chalcid parasitoids. Funding was provided by Agriculture and Agri-Food Canada Project 2955.
Conflict of interest
The authors declare none.











