Introduction
Tebufenozide is an insect growth regulator that was introduced in the early 1990s to control lepidopteran pests (Anonymous 1991). When ingested, it halts larval feeding and induces a developmentally premature moult that is ultimately lethal (Wing et al. Reference Wing, Slawecki and Carlson1988). Tebufenozide is selectively toxic to larval Lepidoptera (Smagghe and Degheele Reference Smagghe and Degheele1994a) and is effective against a range of pests in both agriculture and forestry (Dhadialla et al. Reference Dhadialla, Carlson and Le1998). It is registered in Canada for control of various forest defoliators under the trade name Mimic® (Valent BioSciences Corporation, Libertyville, Illinois, United States of America).
Aerial application of tebufenozide is highly efficacious in suppressing high-density larval populations of eastern spruce budworm (Choristoneura fumiferana (Clemens); Lepidoptera: Tortricidae) (Cadogan et al. Reference Cadogan, Retnakaran and Meating1997, Reference Cadogan, Thompson, Retnakaran, Scharbach, Robinson and Staznik1998, Reference Cadogan, Scharbach, Knowles and Krause2005; Retnakaran et al. Reference Retnakaran, Smith, Tomkins, Primavera, Palli and Payne1997), an economically important defoliator of Canada’s coniferous forests. Several attributes contribute to its efficacy: tebufenozide is highly toxic to spruce budworm (Sundaram et al. Reference Sundaram, Sundaram and Sloane1996, Reference Sundaram, Palli, Ishaaya, Krell and Retnakaran.1998; Retnakaran et al. Reference Retnakaran, Smith, Tomkins, Primavera, Palli and Payne1997; Dallaire et al. Reference Dallaire, Labrecque, Marcotte, Bauce and Delisle2004), is highly resistant to wash-off by rain (Sundaram Reference Sundaram1994), and has extended residual toxicity on foliage (Sundaram et al. Reference Sundaram, Sundaram and Sloane1996). Another key attribute is that tebufenozide appears to suppress spruce budworm populations for some years following application. Anecdotal evidence from operational spray programmes for such multi-year effects (Cadogan et al. Reference Cadogan, Scharbach, Krause and Knowles2002) was corroborated by field trials that demonstrated that aerial application of Mimic® reduced larval populations, defoliation, and egg density not only in the year of treatment but also one or two years following treatment (Cadogan et al. Reference Cadogan, Scharbach, Knowles and Krause2005; Régnière et al. Reference Régnière, Cadogan and Retnakaran2005).
Several mechanisms have been suggested to explain the multi-year effects of tebufenozide.
(1) Underestimation of treatment-induced mortality. Because foliar tebufenozide deposits can retain their toxicity to spruce budworm for two months following spray application (Sundaram et al. Reference Sundaram, Sundaram and Sloane1996), routine assessments of post-spraying larval density, which are typically done within two weeks of application, do not account for the full extent of treatment-induced mortality if additional mortality occurs during late-larval and pupal stages.
(2) Reduced mating success. Tebufenozide can cause severe wing deformities in spruce budworm moths, which can interfere with their mating success (Sundaram et al. Reference Sundaram, Palli, Smagghe, Isayaah, Feng and Primevera2002).
(3) Reduced fecundity. Tebufenozide has been shown to reduce egg production in various Lepidoptera, Coleoptera, and Diptera (Dhadiallia et al. Reference Dhadialla, Carlson and Le1998). Such effects have not been found in laboratory studies with spruce budworm, but those studies used single-dose exposures (Cadogan et al. Reference Cadogan, Scharbach, Krause and Knowles2002; Dallaire et al. Reference Dallaire, Labrecque, Marcotte, Bauce and Delisle2004) whereas, under field conditions larvae are likely to experience chronic exposure due to the long-lasting toxicity of foliar residues.
(4) Reduced fertility. Tebufenozide is known to reduce fertility in many lepidopteran species (see Dallaire et al. Reference Dallaire, Labrecque, Marcotte, Bauce and Delisle2004), but spruce budworm egg hatch was not affected by tebufenozide exposure of larvae in the laboratory or in the field, nor through contamination of egg-laying surfaces or topical contact (Cadogan et al. Reference Cadogan, Scharbach, Krause and Knowles2002; Régnière et al. Reference Régnière, Cadogan and Retnakaran2005).
(5) Reduced early-instar survival. Uptake of tebufenozide by first instars through grazing (Retnakaran et al. Reference Retnakaran, Tomkins, Primevera and Palli1999) and subsequent disruption of moultings was proposed as a key mechanism to explain multi-year effects (Retnakaran et al. Reference Retnakaran, Krell, Feng and Arif2003; Doucet et al. Reference Doucet, Frisco, Cusson, Bauce, Palli and Tomkins2007). However, field application of tebufenozide during moth emergence had no suppressing effect on population density in the year following treatment (Régnière et al. Reference Régnière, Cadogan and Retnakaran2005), indicating that early instars are not directly affected.
(6) Reduced overwintering survival. Tebufenozide exposure broke diapause in larvae of the codling moth, Cydia pomonella (Linnaeus) (Lepidoptera: Tortricidae), by initiating moulting (Sauphanor et al. Reference Sauphanor, Bouvier and Brosse1999). Limited field data suggest that tebufenozide exposure of late-instar spruce budworm larvae could affect overwintering survival of second instars in the next generation (Cadogan et al. Reference Cadogan, Scharbach, Krause and Knowles2002), but data were not conclusive.
Because published data do not yet present a clear picture, we investigated the effects of tebufenozide on spruce budworm survival and performance with the specific intent of bridging the gap between field and laboratory observations. Field data can be confounded by factors that are beyond control of the investigator, and doses and routes of exposure in laboratory experiments often do not relate well to exposures in the field. We therefore exposed spruce budworm larvae under controlled conditions to tebufenozide spray deposits that are representative of aerial field applications, and compared the results of those laboratory experiments to aerial spray trials that were conducted concomitantly.
Materials and methods
Laboratory experiments
Site
Laboratory studies were conducted in 2014 at the Great Lakes Forestry Centre in Sault Ste. Marie, Ontario, Canada. Plots of balsam fir (Abies balsamea Linnaeus; Pinaceae) and white spruce (Picea glauca (Moench) Voss; Pinaceae) established 15 years ago in the laboratory’s compound at a density of ~ 20trees/m2 were used for the experiment with a total of 11 fir plots and eight spruce plots. Height of individual trees in each plot was trimmed to ~ 1.8 m. An enclosure constructed with four vertical sheets of plywood (1.2 m wide×2.8 m high) was placed around each plot during spraying to contain the spray cloud. Sprays were applied when new shoots were fully flushed but not yet elongated.
Tebufenozide treatments
Each plot was treated with a dilution (see below) of the commercial insecticide Mimic® 2LV or with a water-only control, using a hand-held spinning disk applicator (UlvaFan; Micron Inc., Houston, Texas, United States of America). The sprayer was held within the enclosure ~ 30 cm above the canopy for 5–6 seconds, using a product flow rate of 25 mL/minute. In preliminary trials with dyed product, those application parameters resulted in an average deposit of ~ 10 droplets/fir needle, with diameters ranging between 20 and 200 μm (median diameter of 75–80 μm, n=400 droplets). This corresponds to typical droplet size spectra for aerial forestry applications (van Vliet and Picot Reference van Vliet and Picot1987).
Mimic® 2LV (containing 23–25% tebufenozide as per label) was first diluted with water to the recommended field rate (70 g active ingredient (AI) in 2L). Ten product dilutions (the field rate and nine three-fold dilutions) were used to ensure spray deposits that would result in the full range of larval mortality from 0% to 100%, as had been established in preliminary experiments. Each concentration and a water-only control were tested on fir, while a subset of seven concentrations plus the control were tested on spruce. Spray deposits were estimated with artificial foliage-like samplers, as described below (see Tebufenozide spray deposits).
Effects of tebufenozide exposure on survival and development
Effects of tebufenozide treatment on larval survival, development time (days to pupation), pupal mass, pupal survival (adult emergence), and incidence of adult wing deformities were assessed by feeding spruce budworm larvae with treated and untreated foliage in bioassays under controlled laboratory conditions. Twigs with shoots were collected a few hours after spray application and stored in a dark, cold room. Bioassays were conducted in 300-mL cups (Dixie D8; Georgia Pacific Consumer Products, Brampton, Ontario, Canada) containing ~ 15 g of foliage and 10 early fifth-instar spruce budworm from a laboratory colony (Great Lakes Forestry Centre, Sault Ste. Marie, Ontario, Canada) using 10 (in treatments expected to yield 100% mortality) or 20 cups/treatment. Cups were maintained at 21 °C, 60% relative humidity and a 16 light:8 dark hour photoperiod for 14 days until pupation. Foliage was replaced after seven days with foliage from the same plot. Dead larvae were recorded and removed when the foliage was changed and when the bioassay was terminated. Missing larvae were excluded from final analyses as their fate was unknown. Larvae that had pupated were considered survivors. Pupae were harvested daily and pupal mass and sex were determined on the first day of the pupal stage. Pupae were held individually at 12°C and moved to 24 °C once surviving larvae had pupated in all treatments. Adult emergence and severe malformation of adults (heavily crumpled and shortened wings) were recorded.
Effects of tebufenozide exposure on reproduction
Adults produced by tebufenozide-exposed and untreated larvae were used to evaluate the effects of sublethal exposure on mating success, fecundity and fertility. For each concentration treatment with sufficient survivors, up to 30 single-pair matings were set up by placing a one-day-old female and a freshly emerged male in a vented cup containing a balsam fir twig for oviposition (Stehr Reference Stehr1954). After a seven-day oviposition period at 21 °C, 60% relative humidity and a 16 light:8 dark photoperiod (incandescent bulbs), eggs were collected and the mating status of each female was determined by dissection to ascertain the presence of spermatophores. Eggs from each female were placed in a petri dish with a cheese cloth patch on Parafilm® on the inside of the lid, which was then placed in an envelope with a window cut out to entice hatched first instars to spin hibernacula in the cheesecloth patch (Stehr Reference Stehr1954). Realised fecundity was estimated by adding the number of larvae that had spun in to the number that had not, and the number of unhatched eggs in each dish.
Effects on tebufenozide exposure on the next generation
Possible effects of tebufenozide exposure on larvae of the next (F1) generation were assessed using the offspring from 63 females (2743 second instars) from the two highest concentration treatments (dilution 5 fir and dilution 6 fir) that produced offspring (and their control). Cheese-cloth patches containing the offspring from one female were cut in two and one half was used to assess survival after 24 weeks at 4 °C (diapause survival) and the other half to assess survival after 28 weeks in an outdoor insectarium (overwintering survival). Second-instar survival was evaluated as the proportion of larvae that emerged successfully at 21 °C after cold exposure. Post-diapause survival (from emergence to pupation) was evaluated by rearing larvae from the diapause survival experiment in groups of 20–30/cup on an artificial diet (Grisdale Reference Grisdale1984) at 21 °C, 60% relative humidity and a 16 light:8 dark hour photoperiod until the fifth instar. Subsamples of up to 10 larvae/family (for a total of 581 larvae) were then transferred to a fresh diet and reared individually until pupation to assess development time (number of days from second-instar emergence until pupation), pupal fresh weight, and pupal survival (adult emergence).
Field experiments
Aerial spray trials were conducted in 2013 in the Lower St. Lawrence region east of Rimouski, Québec, Canada, an area that was in the early stages of a new outbreak (Pureswaran et al. Reference Pureswaran, De Grandpré, Paré, Taylor, Barrette and Morin2015). Twelve mixed spruce-fir stands of ~ 30 ha with a variable hardwood component were selected along a gradient of larval density. Eight plots were used as untreated controls and four (plots 4, 10, 15, and 24) were treated with Mimic® 2LV (70 g AI in 2.0 L/ha) when larvae had reached the fifth instar. Applications were made by a Cessna 188 fixed-wing aircraft equipped with four Micronair AU4000 atomisers (Micron Sprayers Limited, Bromyard, United Kingdom). The aircraft was guided by Accuair Aerial Management System (McLeod et al. Reference McLeod, Lucarotti, Hennigar, MacLean, Holloway and Cormier2012) to optimise spray deposition. Plot 24 was treated in the morning of 16 June. Plots 4, 10, and 15 were treated in the evening of 18 June and again in the evening of 19 June because the first application was followed by heavy rain. Deposition of tebufenozide was measured on artificial foliage as described below.
Spruce budworm populations were assessed by collecting one 45-cm branch tip from the mid-crown of 30–100 dominant and co-dominant balsam fir and white spruce at the peak of the fourth larval stage (pre-spray), in the late pupal stage (after >50% adult emerge), and after the end of egg hatch. The number of current-year shoots on each branch was determined and insect density was expressed per shoot. Any living larvae and pupae recovered from the late pupal sample were then reared on an artificial diet to determine the number that could have emerged as adults in the field. Current defoliation was estimated on branches collected at the end of the season (after egg hatch) using the method of Fettes (Reference Fettes1950).
Tebufenozide spray deposits
The primary purpose of the spray deposit assessment was to compare tebufenozide exposure achieved in the laboratory to actual field exposure resulting from aerial application. We therefore used the same techniques for collection and quantification of foliar residues in the 2013 field and 2014 laboratory experiments. Spray deposits were collected on sections of artificial Christmas tree branches (Dura Hedge; Discount Fence Supply, Streetsboro, Ohio, United States of America), hereafter referred to as artificial foliage. The sample unit consisted of a section with a length of ~ 15 cm, a mass of ~ 6.4 g, and a surface area of ~ 65.1 cm2. This sample unit is known to have a 50% collection efficiency compared with natural foliage, as was shown in extensive side-by-side comparisons during Mimic® spray trials in New Brunswick (R. Johns, personal communication). In the 2013 field trial, 15 (19 in one plot) trees were randomly selected throughout each sprayed plot, and one sample unit was suspended at mid-crown level of each tree before spray application and removed a few hours after completion of the spray treatment. In the 2014 laboratory experiment, three sample units were placed in the canopy of each 1.2×1.2 m plot and removed after spray application.
Artificial foliage samples that were sprayed in the field were brought to the laboratory and were placed in separate glass vials (Qorpak, Bridgeville, Pennsylvania, United States of America) and tebufenozide was extracted by shaking the sample for 30 minutes in methanol (1×75 mL). The solvent extracts were quantitatively transferred into a round-bottom flask (250 mL) and evaporated to dryness in vacuo on a rotary evaporator (Buchi Model 112, Berlin, Massachusetts, United States of America). Residues were reconstituted in a small volume (2 mL) of 50:50 methanol:water and filtered through a nylon 0.2 μm Acrodisc filter (Gelman Sciences, Montréal, Québec, Canada). The concentrated sample was analysed via high performance liquid chromatography with diode array detection (HPLC-DAD) on an Agilent HPLC 1100 instrument (Agilent Technologies, Santa Clara, California, United States of America) using a Phenomenex Prodigy 5 μm ODS-2 column (150×4.6 mm) (Phenomenex, Torrance, California, United States of America). Separation of analyte from potential co-extractive interferences was achieved with gradient elution, using Pic solution and methanol as the mobile phase, at an initial ratio of 75:25 and rapidly increasing the methanol content. The flow rate was 1 mL/minute and detection wavelength was set to 236 nm. Quality control samples (n=11) were prepared by fortifying blank artificial foliage with known amounts of tebufenozide (0.5–2.0 µg/g fresh weight). Based on the analysis of three replicate samples, the limit of quantitation was estimated at 0.05 µg/g. Mean recovery was 89.3% with a coefficient of variation of 1.1%, indicating substantially higher recovery efficiency and precision as well as lower limits of quantitation compared with similar methods applied to natural foliage samples (Cadogan et al. Reference Cadogan, Thompson, Retnakaran, Scharbach, Robinson and Staznik1998).
Analysis of data
Laboratory experiments
Binomial logistic regression (SAS NLMIXED) was used to determine the effect of host as a factor and concentration C (g AI/L spray solution) as covariate on larval survival, pupal survival, mating success, and egg fertility. Best-fit models were obtained by removing least-significant terms one at a time until the lowest AICc was obtained. The general linear model procedure was used to determine the effect of host and sex (factors) and concentration C as covariate on larval development time, pupal weight, and fecundity. Data from control treatments were incorporated by treating the water control as an additional dilution with near-zero tebufenozide levels.
Field experiment
Survival of spruce budworm from early larval stages to adult emergence is highly density-dependent. A logistic model was used to relate the number of adults in late pupal samples (pupal exuviae plus moths emerged after rearing from collected pupae) on each sample branch tip N to the number of shoots on the branch S, plot-average density of fourth instars (D, larvae/shoot), treatment with Mimic® (C=0 for controls, C=1 for treated plots) and their interaction D×C. The expected adult density (
![]() $\hat{N}$
, adults/branch) is given by:
$\hat{N}$
, adults/branch) is given by:
where parameter p 1 is the maximum adult density (adults/shoot, a carrying capacity related to overcrowding), p 2 and p 4 the intercept and density-dependent steepness for control (untreated) plots, respectively, while p 3 and p 5 are tebufenozide treatment effects on intercept and steepness.
Because spruce budworm distribution on the sampling unit (45-cm branch tip) is known to be aggregated (Régnière and Sanders Reference Régnière and Sanders1983), the negative binomial distribution was used to calculate the likelihood of each adult density observation N (adults or live pupae on each branch):
where k is the negative binomial aggregation parameter. Estimates of the model’s six parameters (p 1–p 5, and k) were obtained using the maximum likelihood with Procedure NLMIXED of SAS/9.2.
Results
Laboratory experiments
Larval and pupal survival
Survival data were fitted to binomial logistic regression models with host (subscript h) as factor and log concentration (C) as covariate:
where c (and c h ) is the maximum survival rate (and host effect), a (and a h ) the intercept of the dose response (and host effect), and b (and b h ) the slope of the dose response (and host effect). Progressive removal of least-significant terms resulted in models (Table 1) predicting larval and pupal survival that were highly correlated with observed survival (r>0.97; Fig. 1). Maximum pupal survival was slightly lower on spruce than on fir across the range of non-lethal dilutions, suggesting a small host effect.

Fig. 1 Observed and predicted spruce budworm (A) larval survival and (B) pupal survival after feeding on foliage of spruce (──●──) or fir (---○---) treated with various dilutions of tebufenozide. Vertical bars: SEM.
Table 1 Estimates and significance of parameters for binomial regression models (see text) that describe the effects of tebufenozide exposure on spruce budworm survival during larval (from early fifth instar to pupation) and pupal stages (equation [3]), mating success, and egg fertility (equation [5]).
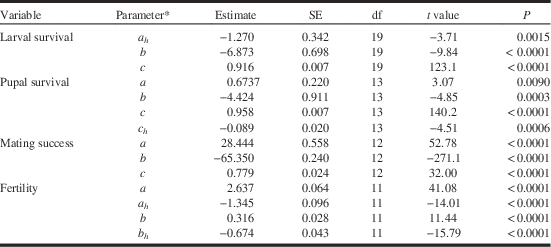
Note:
* c=Maximum rate, a=intercept of dose response, b=slope of dose response, subscript h=host effect.
Larval and pupal development
General linear models were used to determine the effect of host (subscript h) and sex (subscript s) as factors and concentration (C) as covariate on development time from fifth instar to pupation (t) and on pupal weight (W):
where a–d are intercepts and e–h are slopes of the dose response representing host, sex, and interaction effects. Models were simplified by removing least-significant terms one at a time until all remaining terms were significant (α=0.05) (Table 2). Residuals were tested for normality with the Anderson-Darling statistic (AD⩽0.223, df=26, P⩾0.807). There was no effect of host on larval development time (P=0.34), alone or in interaction with other variables (H×C, P=0.557; H×S, P=0.425; H×C×S, P=0.953). Only the main effects of sex and concentration were significant: females took longer to develop than males, and development time was reduced at higher concentrations (Table 2; Fig. 2A; R 2=0.85). The effect of concentration was sex dependent, with females being more affected than males, possibly because more rapid development of males resulted in lower exposure. The weight of pupae was affected by host, sex, and concentration (Table 2; Fig. 2B; R 2=0.87), but not by any of their interactions (H×C, P=0.232; H×S, P=0.407; H×C×S, P=0.81). Pupae were heavier on spruce than on fir, females were heavier than males, and pupal weight decreased with concentration.

Fig. 2 Observed and predicted (A) larval development (days to onset of pupation) and (B) pupal weight (mg) of female (closed symbols) and male (open symbols) spruce budworm after feeding on foliage of spruce (squares) or fir (circles) treated with various dilutions of tebufenozide. Vertical bars: SEM.
Table 2 Main effects of tebufenozide exposure (concentration), sex, tree species (host), and their interactions on spruce budworm larval development time (from early fifth instar to pupation; equation [4]), mass of one-day-old pupae (equation [4]) and number of eggs laid per female (equation [6]), and on larval development (from second-instar emergence to pupation) and pupal mass in the following (F1) generation (equation [8]).
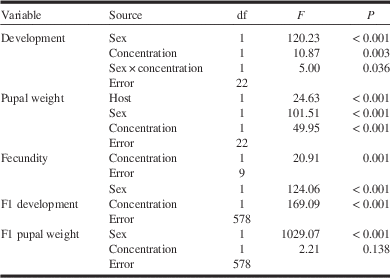
Reproduction
Only 14 of the 1382 adults obtained in these experiments had deformed (crumpled) wings. The proportion of deformed adults did not exceed 5% at any concentration. Deformity was therefore not an important sublethal effect in these experiments.
Effects of tebufenozide exposure on mating success (number of females containing spermatophores, n mated ) and fertility (number of eggs laid by mated females that hatched, n fertile ) were evaluated with a binomial logistic regression model, using host as a factor (subscript h) and concentration (C) as covariate:
where c (and c h ) is the maximum survival rate (and spruce effect), a (and a h ) the intercept of the dose response (and spruce effect), and b (and b h ) the slope of the dose response (and spruce effect). Predicted mating success calculated with the resulting model (Table 1) was highly correlated with observed mating success (r=0.941, P<0.001; Fig. 3A). Mating success dropped sharply among survivors at higher exposures in both host treatments. Fertility of eggs (mated females only) averaged 88%. While all parameters of the model were significantly different from zero (Table 1), the slopes of the dose response were very small and in opposite directions (positive in fir, negative in spruce), indicating that there was really no difference between hosts (87.9% on fir versus 88.5% on spruce).

Fig. 3 Observed and predicted (A) mating success (proportion of females with spermatophores) and (B) fecundity (number of eggs per female) for females that survived exposure to various dilutions of tebufenozide during larval stages on spruce (●) or fir (○). Vertical bars: SEM.
A general linear model was used to determine the effect of host as a factor (subscript h) and concentration (C) as a covariate on fecundity of mated females (F):
where a and b are the intercepts, and c and d the slopes of the dose response representing host and interaction effects. The residuals were tested for normality with the Anderson-Darling statistic (AD=0.235, df=11, P=0.725). There was no effect of host on fecundity. Fecundity dropped at higher concentrations (Table 2; Fig. 3B; R 2=0.67). The fecundity effect may be entirely explained by the effect of tebufenozide on pupal weight of survivors, as weight and fecundity were highly correlated (r=0.879, P<0.001).
F1 survival and development
A binomial logistic regression model with concentration (C) as covariate was used to assess survival of progeny from tebufenozide-treated females:
where a is the intercept and b the slope of the dose response. This analysis was applied to survival from egg hatch to end of cold storage (second-instar emergence), from emergence of second instars to the fifth-instar moult, and from pupation to adult emergence. There was no significant effect of previous-generation exposure to tebufenozide on the survival of F1 larvae during diapause (b=1.0122±0.5867 (SE), Z=1.73, P=0.084) or during the pupal stage (b=−1.1578±1.0251, Z=−1.13, P=0.259). A small (10%) but significant (b=1.0512±0.3179, Z=3.31, P=0.01) increase in post-diapause survival with concentration was caused by low survival in the controls and was probably an experimental artefact. Overwintering survival of second instars during outside exposure could not be analysed because larvae had started to emerge just before cheese-cloth patches were retrieved from the insectarium, resulting in the loss of an unknown number of emerged larvae. Nevertheless, only a very small number of larvae did not emerge successfully in the two concentration treatments and the control, indicating high emergence success and therefore low overwintering mortality in all treatments.
General linear models were used to determine the effect of sex (subscript s) as a factor and concentration (C) as a covariate on development time of F1 larvae from second-instar emergence until pupation (t) and weight of F1 pupae (W):
where a and b are the intercepts and c and d the slopes of the dose response representing sex and interaction effects, respectively. The residuals of both variables were not normally distributed and a log transformation did not help (AD=1.76, df=581, P<0.005), but given the very high F values (Table 2), this non-normality has no influence on the conclusions. The results suggest that tebufenozide exposure of the previous generation shortened larval development time in both males and females (Table 2), whereas weight of F1 pupae was unaffected, regardless of sex (Table 2).
Field trials
The effect of tebufenozide on survival between fourth instar and adult emergence in field populations was determined by fitting the model in equations [1] and [2] to individual branch sample data from the field plots. Five of the model’s six parameters (p
1–p
4 and aggregation k) were highly significant (P<0.0001); Table 3). The interaction term (p
5
DC) in equation [1] was dropped because it did not contribute significantly to goodness of fit (P=0.147). The model fitted the data very well (R
2=0.89; Fig. 4A). Observed survival between the fourth instar and adult emergence (the ratio
![]() $(N/S)/D$
) and its expected value
$(N/S)/D$
) and its expected value
![]() $(\hat{N}/S)/D$
were highly correlated (r=0.9; Fig. 4B). From the parameters of equation [1], mortality inflicted by the tebufenozide treatment M can be calculated as:
$(\hat{N}/S)/D$
were highly correlated (r=0.9; Fig. 4B). From the parameters of equation [1], mortality inflicted by the tebufenozide treatment M can be calculated as:

Fig. 4 Results of the aerial application of tebufenozide in the Lower Saint Lawrence region of Québec in 2013. (A) Relationship between adult density (pupal exuviae and pupae that survived to adult emergence in rearing) and pre-spray (fourth instar) density (lines: equation [1]); (B) Survival from fourth instar to adult (lines: survival predicted with equation [1]); (C) mortality resulting from tebufenozide treatment (line: equation [9]). Controls: ○; treated: ●. Vertical bars: SEM.
Table 3 Parameters of field survival model (equations [1] and [2]).

Predicted values compared very well with observations (r=0.87; Fig. 4C). These results illustrate the strong density dependence of survival from fourth instar to adult in the spruce budworm and the high efficacy of tebufenozide. With an average deposit of 0.76 μg/g, mortality caused by spray treatment averaged 95%. Our analysis suggests that efficacy decreased with increasing population density, with mortality attributed to treatment dropping to 86% in the highest-density population (Fig. 4). We attribute this effect to increased mortality in untreated control populations caused by intraspecific competition at density above 0.25 L4/shoot.
Tebufenozide spray deposits
In the laboratory experiment, tebufenozide spray deposits on artificial foliage samples were analysed in selected treatments only, starting with the lowest concentration that caused 100% larval mortality in the feeding bioassays (dilution 3) and ending with treatments that produced foliar residues approaching the analytical detection limit (dilution 6). Observed foliar residues ranged from 1.45 μg/g (dilution 3 fir) to 0.07 μg/g (dilution 6 spruce, dilution 6 fir; Table 4). Foliar residues were highly variable and sometimes lower than expected (e.g., dilution 4 spruce), probably due to small variations among treatments in atomiser position, flow rate, and spray duration. Field application of Mimic® 2LV resulted in similar tebufenozide deposits on the same artificial foliage samplers, with residues ranging between 0.51 and 1.21 μg/g (Table 4). Residue analyses thus confirmed that the foliar tebufenozide deposits achieved in the laboratory experiments were representative of foliar deposits resulting from aerial application in the field.
Table 4 Tebufenozide spray deposits (μg/g) on artificial foliage samples in the 2013 field trials (Plot) and 2014 laboratory experiments (dilution).
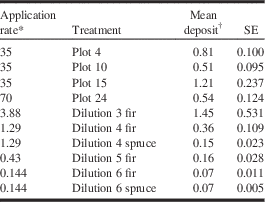
Notes:
* Grams of active ingredient (tebufenozide) per litre of spray solution.
† n=15–19 in field plots; n=3 in laboratory treatments.
Discussion
Application of the commercial forestry insecticide Mimic® 2LV to potted white spruce and balsam fir in outdoor enclosures, followed by bioassays of treated foliage in the laboratory, revealed multiple effects of its AI, tebufenozide, on spruce budworm survival and recruitment. Chronic (14-day) exposure of fifth instars and sixth instars to tebufenozide on foliage reduced larval survival, pupal survival, mating success and fecundity, depending on the spray product concentration that had been applied.
Larval survival declined from ~ 93% at concentrations ⩽0.14 g AI/L to 0% at ⩾3.88 g/L (Fig. 1A). Previous studies have shown that tebufenozide is highly toxic to spruce budworm larvae, with estimates of the 50% lethal dose ranging between 5 and 30 ng/larva, depending on the larval stage and bioassay method (Sundaram et al. Reference Sundaram, Sundaram and Sloane1996, Reference Sundaram, Palli, Ishaaya, Krell and Retnakaran.1998; Retnakaran et al. Reference Retnakaran, Smith, Tomkins, Primavera, Palli and Payne1997; Dallaire et al. Reference Dallaire, Labrecque, Marcotte, Bauce and Delisle2004). Our study shows that chronic exposure of spruce budworm larvae produces significant mortality of pupae as well. Treatments that killed 20–80% of larvae (0.43 and 1.29 g AI/L; Fig. 1A) caused between 15% and 70% mortality of survivors during the pupal stage (Fig. 1B). Expression of delayed mortality during the pupal stage as a result of sublethal larval exposure has been reported for other Lepidoptera as well (e.g., Whiting et al. Reference Whiting, Jamieson and Connolly1999; Biddinger et al. Reference Biddinger, Hull, Huang, Mcpheron and Loyer2006).
Surviving pupae produced adults that had significantly lower mating success (Fig. 3A). Mating success can be affected by tebufenozide-induced wing deformity (Sundaram et al. Reference Sundaram, Palli, Smagghe, Isayaah, Feng and Primevera2002), but that did not play a role in our experiment as the proportion of adults with severe wing deformity was consistently low (5% or less) across treatments. Other factors likely contributed to reduced mating: as an ecdysone agonist, tebufenozide can interfere with physiological mechanisms underlying precopulatory behavioural responses and reproductive processes in ways that are not yet fully understood. For example, sublethal exposure delayed spruce budworm females’ onset time of calling (Dallaire et al. Reference Dallaire, Labrecque, Marcotte, Bauce and Delisle2004); reduced the size and number of spermatophores in treated males of the obliquebanded leafroller (Choristoneura rosaceana Harris; Lepidoptera: Tortricidae) (Dallaire et al. Reference Dallaire, Labrecque, Marcotte, Bauce and Delisle2004); prevented transfer of eupyrene sperms during copulation in treated Helicoverpa zea (Boddie) (Lepidoptera: Noctuidae) (Carpenter and Chandler Reference Carpenter and Chandler1994); and drastically reduced the number of sperm in the upper reproductive tract of treated male Spodoptera litura (Fabricus) (Lepidoptera: Noctuidae) (Seth et al. Reference Seth, Kaur, Rao and Reynolds2004).
Females that mated successfully produced significantly fewer eggs, even at treatment concentrations that had no effects on larval or pupal survival (Fig. 3B). This finding is in contrast with previous studies that reported no effects of tebufenozide exposure on fecundity of spruce budworm (Cadogan et al. Reference Cadogan, Scharbach, Krause and Knowles2002; Dallaire et al. Reference Dallaire, Labrecque, Marcotte, Bauce and Delisle2004). The difference is most likely due to the use of continuous exposure in our experiment versus single-dose exposure in the others. Some of the effect of sublethal tebufenozide exposure on fecundity is explained by its effect on pupal weight (Fig. 2B), as female pupal weight and fecundity were highly correlated, but chronic exposure may also directly reduce spruce budworm fecundity by interfering with ovarian maturation, as was reported for the closely related C. rosaceana (Sun et al. Reference Sun, Barrett and Biddinger2000; Dallaire et al. Reference Dallaire, Labrecque, Marcotte, Bauce and Delisle2004), as well as other Lepidoptera (Smagghe and Degheele Reference Smagghe and Degheele1994a; Salem et al. Reference Salem, Smagghe and Degheele1997).
We believe that the multiple effects we observed in the laboratory are likely to occur in the field following aerial application of Mimic® 2LV. Results of our deposit assessments (Table 4) suggest that tebufenozide exposure in our laboratory experiment was representative of exposure experienced by spruce budworm larvae in treated field populations. Treatment with product concentrations (0.14–3.88 g AI/L) that affected spruce budworm performance in the laboratory trial produced spray deposits between 0.07 and 1.45 μg tebufenozide/g of foliage, with the latter causing 100% of late-instar mortality. Similar levels of larval mortality were obtained by Sundaram et al. (Reference Sundaram, Sundaram and Sloane1996) in bioassays of foliage with 1.5 μg tebufenozide/g. In comparison, average deposits observed in our field trials after aerial application ranged between 0.5 and 1.2 μg/g (Table 4), with an average deposit of 0.76 μg/g causing 95% mortality (Fig. 4). It can be argued that exposure to foliar residues that presumably degraded little during 14 days of larval feeding in the laboratory is likely to overestimate exposure in the field where spray deposits are subjected to continuous degradation. However, average deposits resulting from aerial application can be as high as 6–7 μg/g of foliage (Sundaram et al. Reference Sundaram, Sundaram and Sloane1996; Cadogan et al. Reference Cadogan, Thompson, Retnakaran, Scharbach, Robinson and Staznik1998), and have an estimated half-life of at least three weeks (Sundaram et al. Reference Sundaram, Sundaram and Sloane1996). This means that larvae feeding during the few weeks between spray application and pupation are likely to experience chronic exposure that is comparable to our laboratory exposure. The decay curve of foliar tebufenozide residues under field conditions (Fig. 5), which we constructed from data published by Sundaram et al. (Reference Sundaram, Sundaram and Sloane1996), shows that tebufenozide deposits that produce adverse effects at various stages of the spruce budworm life cycle in our laboratory trial represent foliar residues along the entire period of persistence in the field, which is over 60 days. Foliar residues at the end of the decay curve are still high enough to affect mating success and fecundity. Together, these data suggest that during two to three weeks of post-spray feeding in the field, larvae likely experience chronic exposure to foliar residues in a range that causes the full spectrum of effects described in this study.

Fig. 5 Decay curve of tebufenozide residues on white spruce foliage after simulated aerial application (calculated from the field data published by Sundaram et al. Reference Sundaram, Sundaram and Sloane1996).
Results of our experiments suggest that reduced survival during late-larval and pupal stages combined with lower recruitment as a result of reduced mating success and fecundity of survivors may play a role in the reported effects of Mimic® 2LV on spruce budworm populations in the year following treatment. The contributions of several other factors can be ruled out. Although tebufenozide is known to reduce fertility in many Lepidoptera species (as summarised in Dallaire et al. Reference Dallaire, Labrecque, Marcotte, Bauce and Delisle2004), results of our experiments corroborate previous observations that tebufenozide exposure does not affect spruce budworm egg hatch in the laboratory (Cadogan et al. Reference Cadogan, Scharbach, Krause and Knowles2002) nor in the field (Régnière et al. Reference Régnière, Cadogan and Retnakaran2005). Neither do our data support the hypothesis that tebufenozide reduces early-instar survival, as was evident from the total lack of exposure-related reductions in second-instar emergence after completion of diapause, as well as from partial evidence indicating no effect on overwintering mortality. Furthermore, we tested the hypothesis that tebufenozide exposure affects performance (survival, development) of spruce budworm larvae in the next generation, which was not the case either (Table 2). Multiple effects of chronic tebufenozide exposure of larvae that are expressed during later stages of the pest’s life cycle, as observed in this study with spruce budworm, have been reported for other target pests as well (Rodriguez et al. Reference Rodriguez, Reagan and Ottea2001; Biddinger et al. Reference Biddinger, Hull, Huang, Mcpheron and Loyer2006). Such effects are believed to explain enduring population control of Diatraea saccharalis (Fabricius) (Lepidoptera: Pyralidae) achieved with large-scale aerial application of Mimic® in sugar cane fields (Rodriguez et al. Reference Rodriguez, Woolwine, Ostheimer, Schexnayder, Reagan and White1998, Reference Rodriguez, Reagan and Ottea2001).
Chronic exposure appears to be key to tebufenozide efficacy. Being an ecdysone agonist, its effect is highly dependent on ecdysone titres in larvae at the time of ingestion. Using single-dose exposure, Palli et al. (Reference Palli, Primavera, Tomkins, Lambert and Retnakaran1995) demonstrated that tebufenozide induced lethal moults in 100% of spruce budworm larvae only when ingested well before the appearance of the endogenous moult-inducing ecdysteroid peak (specifically, before day three in the fifth instar and before day four in the sixth instar). Smagghe and Degheele (Reference Smagghe and Degheele1994b) reported that tebufenozide is rapidly excreted from the digestive system, at least in Spodoptera Guenée species. This means larvae need to repeatedly ingest the compound over time to ensure exposure at low endogenous ecdysone levels, which happens because foliar residues remain toxic for weeks if not months following spray application (Sundaram et al. Reference Sundaram, Sundaram and Sloane1996; Smirle et al. Reference Smirle, Lowery and Zurowski2004). Because of this ecdysone-titre dependency, chronic exposure is critical for proper evaluation of how tebufenozide affects the target pest. Dallaire et al. (Reference Dallaire, Labrecque, Marcotte, Bauce and Delisle2004) reported few sublethal effects of tebufenozide when C. rosaceana larvae were given a one-time dose through force-feeding compared with larvae that were chronically exposed in their diet. The need to repeatedly ingest the insecticide over time may also explain why bioassays that use single-dose exposures typically produce poor dose-response regressions (e.g., Retnakaran et al. Reference Retnakaran, Smith, Tomkins, Primavera, Palli and Payne1997; K.v.F., unpublished data). We suspect that many discrepancies in published data on how tebufenozide affects larval Lepidoptera can be attributed to differences in methods, duration, and level of exposure.
In conclusion, our field and laboratory data confirm that tebufenozide is highly efficacious against spruce budworm. Foliar deposits in the range of ~ 0.5–1.5 μg AI/g caused high levels of larval mortality. Sublethal larval exposure caused delayed mortality during the pupal stage and reduced mating success of survivors in the ~ 0.15–0.5 μg/g range. Sublethal exposure to levels between 0.07 and 0.15 μg/g reduced fecundity of mated females. Our deposit data confirm that such exposure is likely to occur in the field following spray application of Mimic®. Sublethal larval exposure did not affect egg fertility, survival of early instars during diapause, or post-diapause survival of larvae and pupae. Reduced survival during late-larval and pupal stages combined with lower recruitment as a result of reduced mating success and fecundity of survivors are likely to play a role in the suppression of Mimic®-treated spruce budworm populations in the years following treatment. The large reduction of population density resulting from a Mimic® application is likely to trigger increased mortality from natural factors in the following generations, as a result of the density-dependent survival relationship found in our field trial (Fig. 4B). Thus, it may well be the response of spruce budworm’s natural population regulation mechanisms that actually causes the prolonged population reduction effect of tebufenozide.
Acknowledgements
This work was carried out with financial help from Forest Protection Limited (Fredericton, New Brunswick) and other members of Spray Efficacy Research Group International (provincial forestry departments of Newfoundland and Labrador, Nova Scotia, Québec, Ontario, Manitoba, and Saskatchewan; Société de Protection des Forêts contre les Insectes et Maladies and United States Department of Agricuture Forest Service). The Atlantic Canada Opportunities Agency and the Canadian Forest Service contributed considerable funding and support. The authors thank Valent BioSciences Corporation for financial support and supplying Mimic® 2LV, and Forest Protection Limited for conducting the aerial applications. Many are owed thanks for technical support, in particular Ariane Béchard for running the field programme, Yuehong Liu and Brooke Janzen for conducting the laboratory experiments, and Derek Chartrand for doing the tebufenozide residue analyses.











