Introduction
Regional species lists may be used to prepare species status assessment reports and to inform conservation efforts and environmental assessment studies (Catling et al. Reference Catling, Packer and Goit2009; Robinson et al. Reference Robinson, Fordyce and Parker2016), but time and effort is needed to keep them up to date. For insects, including aquatic insects, these lists may be compiled from a combination of new sampling and checklists from published sources (e.g., Robinson et al. Reference Robinson, Fordyce and Parker2016). The Biological Survey of Canada (BSC) has been facilitating collection of species information in Canada for 40 years (since 1977; Danks Reference Danks2016) and in that time, taxonomic tools for identification of organisms have advanced greatly. These include advances in online databases and geographic tools, improved access to new taxonomic revisions and keys as well as older taxonomic literature (see discussions in Guerra García et al. Reference Guerra García, Espinosa Torre and García Gómez2008; Tancoigne and Dubois Reference Tancoigne and Dubois2013) and molecular methods like DNA barcoding (e.g., Ball et al. Reference Ball, Hebert, Burian and Webb2005; Webb et al. Reference Webb, Jacobus, Funk, Zhou, Kondratieff and Geraci2012). Although new sampling is usually preferred when carrying out regional biological surveys, the cost of such surveys may be prohibitive (Robinson et al. Reference Robinson, Fordyce and Parker2016), particularly for isolated areas such as the Canadian North (Cordero et al. Reference Cordero, Sánchez-Ramírez and Currie2016). Where available, ecological and species survey data from the literature can provide valuable baseline data and extend the information on species in a cost-effective manner (e.g., Robinson et al. Reference Robinson, Fordyce and Parker2016). How useful are these lists for Ephemeroptera species, and what factors must be considered to assess their use?
Challenges to using published species lists (e.g., from ecological surveys) generally focus on data quality (Stribling et al. Reference Stribling, Moulton and Lester2003), including questions about correct identifications and validity of older lists following taxonomic revisions (Patterson et al. Reference Patterson, Mozzherin, Shorthouse and Thessen2016). Further, ecological studies may not identify specimens to species (due to a combination of resources and inappropriate life stages for identification), so the data are limited in their use for species assessments (Stribling et al. Reference Stribling, Moulton and Lester2003). Some discrepancies in species lists can be resolved easily, simply by updating names that have been synonymised or transferred to different genera. However, taxa that have undergone significant revision, such as the Ephemeroptera family Baetidae, include new species and many new combinations as well as synonymies. For example, since the publication of the first comprehensive key to Baetis Leach species by Morihara and McCafferty (Reference Morihara and McCafferty1979), several genera have been split from Baetis (e.g., McCafferty and Waltz Reference McCafferty and Waltz1995; McCafferty et al. Reference McCafferty, Meyer, Randolph and Webb2008) and species from other baetid genera have been reassigned to several genera, including Baetis (McCafferty and Waltz Reference McCafferty and Waltz1990). The most recent key to Baetis species (Wiersema et al. Reference Wiersema, Nelson and Kuehnl2004) is also out of date, as new species descriptions and synonymies continue to be published (e.g., Webb Reference Webb2013; Jacobus and Wiersema Reference Jacobus and Wiersema2014). Although historical studies provide a rich source of biodiversity information (and may also include important ecological context for species occurrences), specimens may need to be re-examined to ensure accurate and valid species lists from the study. Re-examination of material from historical studies also assumes that specimens have been archived in accessible collections, stored in a fashion that prevents or reduces deterioration, and are associated with readable label information.
The extensive Mackenzie River Pipeline study of 1971–1973 (Brunskill et al. Reference Brunskill, Rosenberg, Snow, Vascotto and Wageman1973) provides a good example of an historical study, which can be used to update regional biodiversity information for a poorly known region in the Canadian north. The study was designed to examine potential impacts of construction and operation of an oil pipeline along the Mackenzie River, and resulted in thousands of aquatic insect samples from otherwise inaccessible and isolated areas being archived in government-held collections. Taxa lists from the study were published in Wiens et al. (Reference Wiens, Rosenberg and Snow1975) and Cobb and Flannagan (Reference Cobb and Flannagan1980), but they list relatively few Ephemeroptera species and include many problematic generic names. In this study, we explore the benefits and challenges of updating the regional Ephemeroptera species list for the Northwest Territories and northern Yukon by re-examining archived specimens. We also compare the benefits (in terms of species composition and diversity information) from the archived specimens to data from more recent sampling in the region.
Methods
Specimens examined for this study came from two sources: (1) historically archived collections from the Mackenzie Pipeline study (Porcupine River Drainage) in northern Yukon, and Mackenzie Valley tributaries in Northwest Territories (Brunskill et al. Reference Brunskill, Rosenberg, Snow, Vascotto and Wageman1973; Wiens et al. Reference Wiens, Rosenberg and Snow1975) and (2) from more recent collecting in Yukon in 2006, along the Horton and Thelon rivers (described in Currie et al. Reference Currie, Giberson and Brown2000, Reference Currie, Giberson and Adler2002, respectively), Mackenzie River tributaries (Rempel and Gill Reference Rempel and Gill2010; Vinke et al. Reference Vinke, Medeiros and Giberson2015), and additional sites in Yukon and near Yellowknife, Norman Wells and Banks Island, Northwest Territories (Cordero et al. Reference Cordero, Sánchez-Ramírez and Currie2016) (Fig. 1).
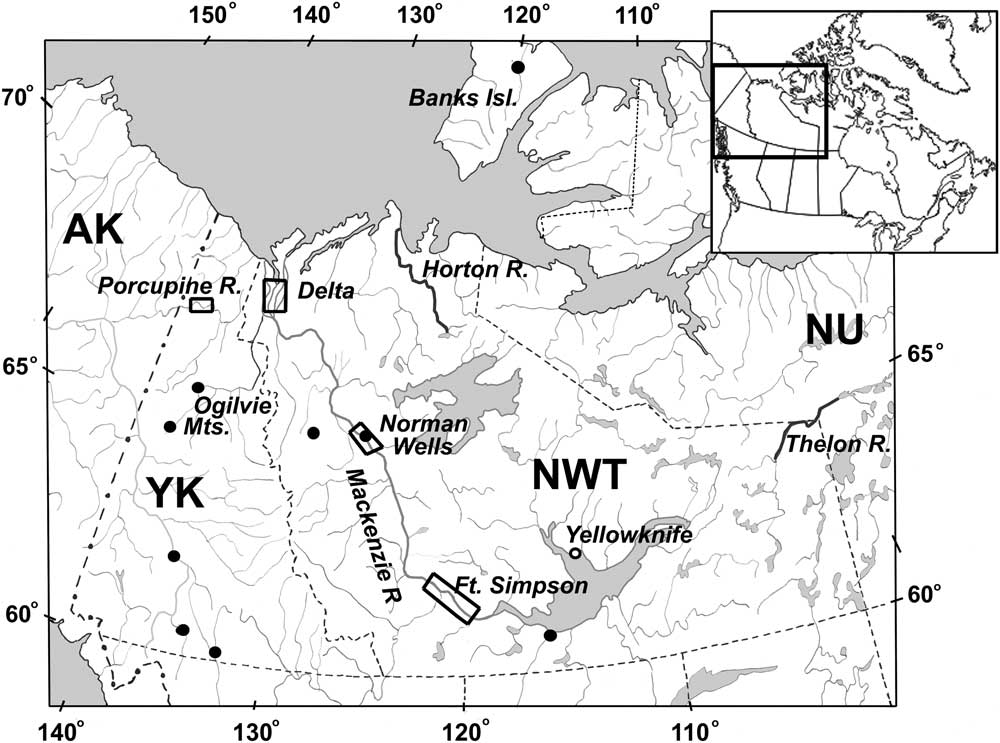
Fig. 1 Yukon and Northwest Territories, showing locations of study areas sampled in this study. Open rectangles indicate the study areas from the 1971–1973 Mackenzie Valley pipeline study, and closed circles indicate the sampling sites visited in collecting trips since 2000. Tributaries and the wadeable sections of the main rivers were sampled along the entire river corridor in 2005 and 2006 for the Mackenzie River by L. Rempel (Rempel and Gill Reference Rempel and Gill2010) and for the Horton and Thelon rivers (Currie et al. Reference Currie, Giberson and Brown2000, Reference Currie, Giberson and Adler2002), so individual sampling sites are not indicated for these studies. Southern Yukon sites were sampled in 2006, and Ogilvie Mountain sites (along the Dempster Highway) were sampled in 2006 and again in 2010 by Cordero et al. (Reference Cordero, Sánchez-Ramírez and Currie2016). Inset: map of Canada showing study region. AK, Alaska; YK, Yukon; NWT, Northwest Territories; NU, Nunuvut.
Archived Mackenzie River pipeline study samples: The Mackenzie River study was carried out by researchers from the Freshwater Institute of Fisheries and Oceans Canada (Winnipeg, Manitoba, Canada), and methods and collecting localities are described in Brunskill et al. (Reference Brunskill, Rosenberg, Snow, Vascotto and Wageman1973). Aquatic invertebrates were collected from rivers, streams, and lakes along proposed pipeline routes from northern Yukon (Porcupine River Drainage) and the Mackenzie River Delta, to the southern portions of the Mackenzie River near Norman Wells and Fort Simpson, Northwest Territories (Fig. 1). Their goal was to assess taxonomic composition, seasonal and spatial diversity and abundance, effects of sediment addition on benthos, and effects of oil on benthos. The diversity of habitats and many study questions resulted in a wide diversity of sampling methods including Surber and kick sampling (small streams and river edges), drift nets and artificial substrate boxes (small-sized to medium-sized streams), floating baskets (large rivers), Ekman dredges (large rivers, lakes, ponds), dip nets (lake and river littoral samples), and pan traps (passive streamside adult collection at all sites). Samples were collected in all seasons, preserved in formalin, and later washed into 70% ethanol for sorting and identification. Samples were sent to the Biosystematics Research Institute in Ottawa, Ontario, Canada (now the Canadian National Collection of Insects) for identification, and a subset of these were retained at the Canadian National Collection of Insects. Remaining vials were returned to the Freshwater Institute. They were then archived in storage until 2009, when pressure to clean out the vial room to make room for other uses prompted re-examination of the specimens for this study. In 2010, Mackenzie Study Ephemeroptera vials from Freshwater Institute and the Canadian National Collection of Insects were combined to give ~2930 vials representing 588 different sites and dates for re-examination. Some vials were broken or misplaced over the years, and others had dried, but 12 215 specimens were available for re-examination for this study.
Recent collecting in Yukon and Northwest Territories: The more recent collections were garnered from five collecting trips/studies spanning the period between 2000 and 2011 (Fig. 1).
-
(1) Barrenland rivers in Northwest Territories, 2000–2002: The Horton and Thelon rivers (and their tributaries) were sampled in summer (June–July) 2000 and 2002, respectively, by travelling down them by canoe, and stopping at intervals to sample the main rivers and tributaries (Currie et al. Reference Currie, Giberson and Brown2000, Reference Currie, Giberson and Adler2002). Insects were collected in wadeable areas using kick/dip nets and along the stream edges using an aerial net, rock searches, and Malaise trapping. Mature specimens were sorted visually on site.
-
(2) Mackenzie River Biomonitoring Project, 2005–2006: 102 sites (tributaries and mainstem) along the Mackenzie River from Great Slave Lake to the Delta were sampled by kick net (wadeable streams) and ponar grab (large rivers) in early fall (late August/early September) and preserved for later sorting as part of a biomonitoring programme led by Department of Fisheries and Oceans Canada scientist Laura Rempel, and Ephemeroptera specimens were made available for this study (Rempel and Gill Reference Rempel and Gill2010).
-
(3) Yukon streams, 2006: streams along the Klondike Highway near Whitehorse and in the Ogilvie Mountains along the Dempster Highway to the Yukon/Northwest Territories border were sampled by kick net and sorted on site in July 2006.
-
(4) Norman Wells biomonitoring study, 2010–2011: Samples were collected monthly between June and October by kick/dip net in streams and adjacent ponds near Norman Wells, supplemented by aerial collections and Malaise trapping, in cooperation with the Sahtu Renewable Resources Board (Vinke et al. Reference Vinke, Medeiros and Giberson2015).
-
(5) Northern Biodiversity Programme, 2010–2011: samples were collected from aquatic habitats near Yellowknife, Norman Wells, and Banks Island and sorted on site. A subsample of these was submitted to the Biodiversity Institute of Ontario, University of Guelph (Guelph, Ontario, Canada) for DNA barcoding (Buddle et al. Reference Buddle, Currie and Giberson2008; Cordero et al. Reference Cordero, Sánchez-Ramírez and Currie2016).
All specimens were collected into 80% ethanol, except for those collected for the barcoding study (preserved in 95% ethanol) and by L. Rempel (collected into 10% formalin, then washed into 70% ethanol for processing). Approximately 1500 specimens from these recent collections were large and intact enough to identify at least to genus.
Working with archived samples: The archived samples from the 1970s Mackenzie River pipeline study were generally in good condition, though some were in discoloured ethanol due to bleeding from the rubber stopper, or contaminated with residual oil from oil spill experiments in the original study. A major challenge to working with these specimens was the interpretation of coded labels (Fig. 2) from the ecological study, which was a necessary first step to validating samples for biodiversity assessment. Labels in all the vials from this study contained at least a site/date/sample code (Fig. 2A), and only about 20% had labels with additional site or date information, though many of these were too degraded to read (Fig. 2B). Family determinations were generally written on the front or back of the labels, occasionally supplemented by a genus or species determination, often with the name and date of the person who identified the specimens in that vial (Fig. 2C). More frequently, specimens that could be identified to genus or species were isolated from the rest of the vial contents in micro-vials, and each micro-vial had its own determination label (Fig. 2C). There were usually multiple species or genera in individual vials. Information to decipher the coded labels was found in Brunskill et al. (Reference Brunskill, Rosenberg, Snow, Vascotto and Wageman1973), but was not clearly indicated, and required considerable searching through the 472-page document and consultation with the original researchers. The breakdown of the code is shown in Figure 2D.
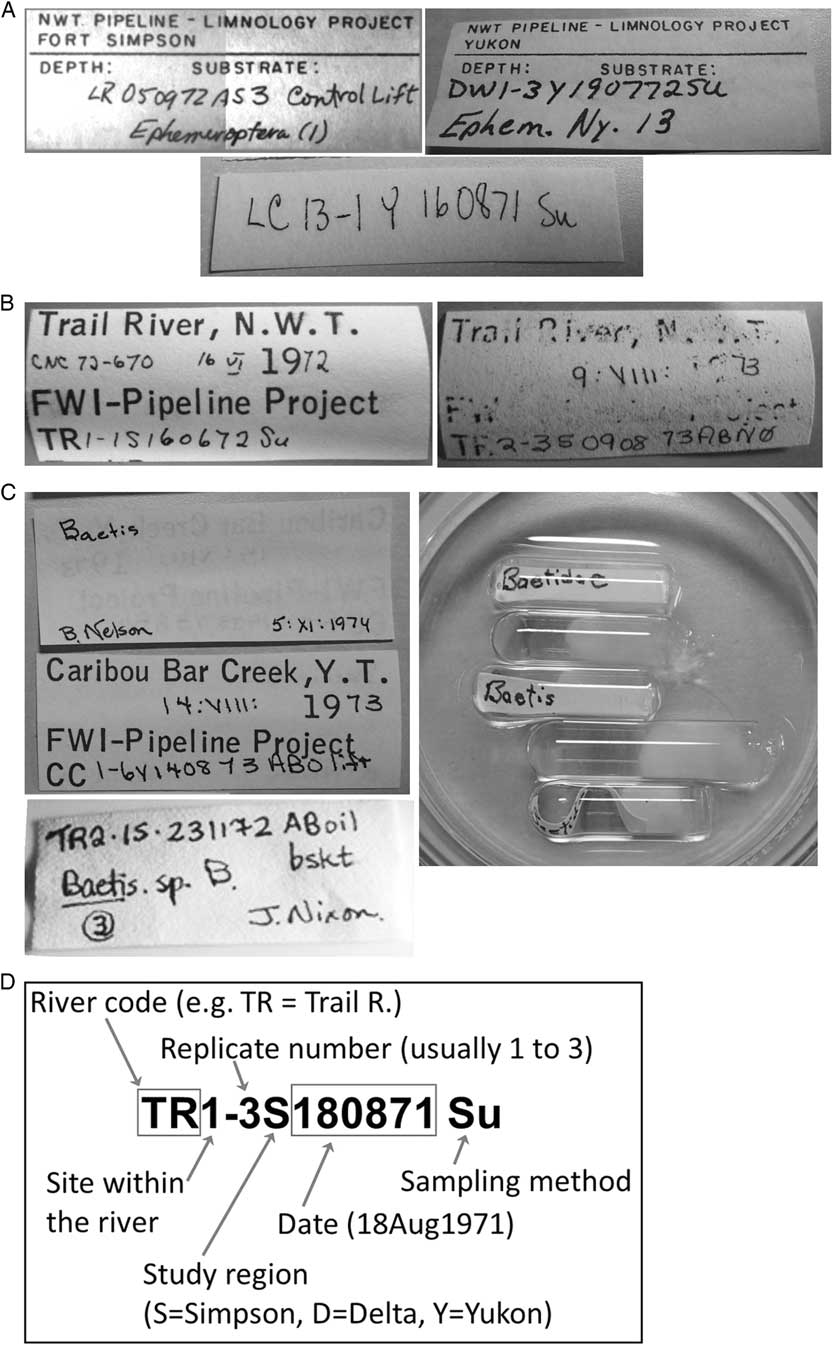
Fig. 2 Sample labels from Mackenzie pipeline study. A. Sample coded labels of the type found in most vials. B. Labels showing location and date information as well as the standard codes; many of these were badly degraded as shown in the label at the right. C. Samples of taxa determination labels, showing the determination on the back of a site label, on the front of the label with the code, and on tiny labels placed into micro-vials. D. Example of how labels were decoded using Brunskill et al. (Reference Brunskill, Rosenberg, Snow, Vascotto and Wageman1973).
Specimen processing: Once labels were decoded, vials were sorted and grouped by site, replicate, and date, and the vial contents were examined and identified. Specimens were identified to genus using Waltz and Burian (Reference Waltz and Burian2008), updated with couplet “patches” to reflect new taxonomic treatments of various genera (e.g., Jacobus and Wiersema Reference Jacobus and Wiersema2014). Specimens that were mature and intact enough to identify to species were separated, then identified by consulting current revisions for individual genera, and comparing characteristics to original species descriptions and identified and verified reference material held at the Northeast Ephemeroptera Laboratory Research Collection at the Department of Biology, Southern Connecticut State University (New Haven, Connecticut, United States of America). The abbreviation “cf.” is used to indicate that the specimen(s) examined share many character states with the species named but may actually represent a different taxon.
Updated identifications from the Mackenzie River pipeline study were compared to the original label determinations, to published species lists for the project, and to mayflies collected since 2000, to assess the benefits of re-examining the historic material in assessing regional biodiversity in the area.
Results
Despite the age of the archived specimens, many of the larger specimens were in good enough shape to identify, at least to genus. Taxa determination labels from the 1970s that were in the vials generally matched up well with the taxa lists published in Wiens et al. (Reference Wiens, Rosenberg and Snow1975) and Cobb and Flannagan (Reference Cobb and Flannagan1980), suggesting that the vials re-examined were a good representation of the original study material.
Nearly 70% of the specimens (8353 of 12 215) were identified only to the family level in the 1970s study (Table 1). Many of these were very small, so even with improved taxonomic resources, it was still not possible to identify the majority of them (6212 specimens) further than family. However, an additional 2141 specimens (a further 18%) could be identified at least to genus in the current study (Table 1), thanks to the availability of updated taxonomic resources (keys and revisionary works) and improved understanding of how the older taxonomic literature related to the current species characteristics. In the 1970s study, only 176 specimens were identified to species (52 of which are still current and were correctly identified; Table 1), resulting in a list of 17 species (six of which are still correct) (Tables 2–3). In the current study, 2272 specimens were identifiable to species, giving 45 species in total. When these were added to specimens from our “new” (post-2000) sampling in the region, 74 species of mayfly were identified from Yukon and the Northwest Territories (Tables 2, 3).
Table 1 Total specimens that could be identified to family, genus, or species, compared between original vial labels and re-examined specimens.

Note: For “pipeline labels”, numbers indicate whether the identification on the old labels was still correct or current (Corr) or were misidentified, represented names that have been synonymised, superseded, or were otherwise invalid, or where no determination label was present (Inval) (see details within each family). “cf.sp.” refers to tentative species identifications. Where the only change was a subgenus name that has been elevated to genus (but was otherwise correct), label identification was considered to be correct (e.g., Ephemerella bicolor=Eurylophella bicolor, Heptagenia hebe=Leucrocuta hebe, and Stenonema vicarium=Maccaffertium vicarium).
Table 2 Numbers of genera and species listed on original pipeline study labels (Yukon and Northwest Territories combined) compared to those resulting from the re-examination of the specimens and new collections for this study.
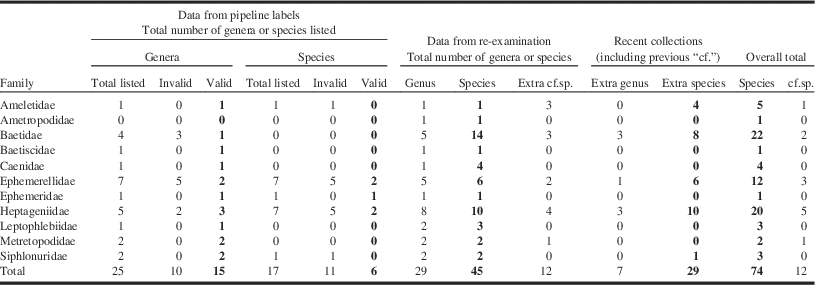
Note: “Pipeline label” data columns indicate total listed as well as those found to be incorrect or represented names that have been synonymised, superseded, or otherwise invalid (Inval) (Table 1 for details within each family). The “cf.sp.” columns refer to tentative species. Numbers in bold text represent total valid species.
Table 3 Comparison of taxa lists generated from the original label data from Mackenzie Valley pipeline vials, the updated information following re-examination of specimens, and new (post-2000) sampling in Yukon and Northwest Territories, Canada (see Fig. 1 for collecting localities).

Notes: Total number of specimens is given for all collections except Cordero et al. Reference Cordero, Sánchez-Ramírez and Currie2016, where “x” denotes presence based on barcode analysis. Deeply indented entries in the first column indicate misidentifications or invalid names from the original pipeline study labels and how they were corrected.
Literature records: 1Mayfly Central (2016), given as “Canada far north”; 2Cobb et al. (Reference Cobb, Flannagan, Flannagan and Wickstrom1995); 3Cobb and Flannagan (Reference Cobb and Flannagan1980); 4Cordero et al. (Reference Cordero, Sánchez-Ramírez and Currie2016); 5Funk and Sweeney (Reference Funk and Sweeney1994); 6Gorski et al. (Reference Gorski, Fox, McQueen and Jacobus2014); 7Harper and Harper (Reference Harper and Harper1981); 8Harper and Harper (Reference Harper and Harper1997); 7Moore (Reference Moore1977); 10Randolph (Reference Randolph2002); 11Wiens et al. (Reference Wiens, Rosenberg and Snow1975); 12Zloty (Reference Zloty1996); 13McCafferty et al. (Reference McCafferty, Meyer, Webb and Jacobus2004); 14Vinke et al. (Reference Vinke, Medeiros and Giberson2015).
Territorial records: NR, new record; NR?, potential new record, but species identification is tentative only. YK, Yukon; NWT, Northwest Territories.
Identifications: The abbreviation “cf.” is used to indicate that the specimen(s) examined share many character states with the species named but may actually represent a different taxon. A species or genus name followed by a question mark (?) represents a tentative identification where specimens were too small for positive identification, but the identification is likely correct.
Comparing original 1970s pipeline study label data with updated identifications
Genus-level identifications: A total of 3633 specimens were originally identified to genus, and of these, a little over half were correctly placed into a genus that is still valid taxonomically (including those that could be further identified to species) (Tables 1, 3). All or most of the specimens identified to genus were found to still be correctly assigned to that genus in the families Ameletidae, Baetiscidae, Caenidae, Ephemeridae, Metretopodidae, and Siphlonuridae (see Table 3 for details within each family). However, taxonomic revisions in the Baetidae, Ephemerellidae, and Heptageniidae resulted in many of the specimens formerly placed in large “catch-all” genera (like Baetis, Ephemerella Walsh, and Heptagenia Walsh) to now be placed in new or different genera (Table 3). This was particularly true in the Baetidae, the family that made up nearly three-quarters of the mayflies examined.
For Baetidae and Heptageniidae, it was necessary to physically examine specimens to update the list of genera, since genus names used in the 1970s survey could not be used to predict the groupings of genera identified using updated taxonomic resources. This was particularly true for the Baetidae, as specimens did not fit into discrete new genera derived from previously named genera. For example, specimens originally labelled “Pseudocloeon sp.” (a genus no longer recognised in North America) were re-identified as species of Acentrella Bengtsson, Acerpenna Waltz and McCafferty, or Plauditus Lugo-Ortiz and McCafferty. Similarly, those previously identified as “Centroptilum sp.” were updated as species in Acentrella or Baetis Leach, and those previously identified as “Baetis sp.” were identified as species in Acentrella, Acerpenna, Baetis, Plauditus, or Procloeon Bengtsson. However, there have been no changes in genera for groups such as Ameletus Eaton or Caenis Stephens, so these showed no change between the 1970s and the current study.
Species-level identifications: Of the 176 specimens originally identified to species (based on presence of species determination labels in the vials), only 52 of these still had current names or were identified correctly (Tables 1, 3). This compares to 2272 that could be identified to species in the current study. However, the success of updated species identification varied widely by family, depending on both the state and size of the specimens and on the availability of updated taxonomic resources. All, or nearly all, the specimens in Ametropodidae, Baetiscidae, Caenidae, and Metretopodidae could be now be identified to species, about three-quarters of the Ephemeridae could be identified to species, and about half of the Leptophlebiidae and Siphlonuridae could be identified to species. In contrast, only 4, 13, and 19% of ameletid, baetid, and heptageniid specimens (respectively) could be identified to species after re-examination, though two of these were also the families that produced the greatest number of additional species during re-examination: 14 baetid species and 10 heptageniid species. The low percentage “success” rate in these groups was mainly due to the small size of the specimens: the original study collected bulk samples from many rivers over multiple years and seasons, giving a variety of size classes of specimens. The old specimens were also very faded, which made it difficult to identify groups where colour patterns were important characters for identification, such as the Ameletidae.
Comparison with “new” sampling expeditions to Yukon and Northwest Territories
Nearly 1500 specimens from the five additional sampling expeditions that included parts of Yukon and the Northwest Territories since 2000 were also examined and identified. These had the advantage of being newer, showing clear colour patterns and being morphologically intact, and in the case of the Northern Biodiversity Programme specimens, preserved specifically to allow DNA barcoding. Despite the relatively low numbers of specimens compared to the Mackenzie Valley pipeline study samples (1495 compared with 12 215), the number of species in Yukon and Northwest Territories identified from the two sets of samples were similar (45 in the pipeline samples compared to 50 in the newly collected samples) (Table 3). There was some overlap in species composition, so the newer sampling added 29 additional species when compared to the pipeline study samples. When the lists were combined, the total climbed to 74 species (plus several tentative, “cf.” identifications).
Discussion
The clear benefits in updating and clarifying previously published species lists from re-examining archived Mackenzie Valley pipeline study samples fully justified the challenges encountered in dealing with the samples. It was difficult and time consuming to locate the sample vials associated with this study and gather them into a single location so that the study could proceed, and it is likely that some were missed in the storage areas at the Freshwater Institute (Winnipeg) and the Canadian National Collection of Insects (Ottawa). Some vials had dried out, and could not be rehydrated. Further, lack of clear information on original specimen labels (including those deposited at the Canadian National Collection of Insects) was a major drawback to implementing this study, and required considerable research to resolve. However, the archived samples gave access to specimens over a vast geographical area from southern Northwest Territories (~60°N) to the Mackenzie Delta (to 68.5°N) and west to northern Yukon (Brunskill et al. Reference Brunskill, Rosenberg, Snow, Vascotto and Wageman1973), and over a variety of seasons and habitats that would be difficult and very expensive to sample today. Another major benefit was that samples were already sorted from the sample debris, resulting in a major cost savings in processing samples.
Re-examining the archived samples provided new data on relative abundance and distributional data for 45 species, several of which were not previously reported in published accounts for Yukon (seven species, plus two tentative (cf.) species) or the Northwest Territories (14 species plus three tentative (cf.) species; these are in addition to new records from this study already published in Vinke et al. Reference Vinke, Medeiros and Giberson2015; Cordero et al. Reference Cordero, Sánchez-Ramírez and Currie2016). Nearly all of these required re-examination of original specimens to detect. An example of updated distributional data includes the records from river sites for Ameletus inopinatus Eaton (Ephemeroptera: Ameletidae) (previously identified as “Ameletus sp.”). These expand the previously restricted habitat association in northern Canada (i.e., lakes and ponds, Zloty Reference Zloty1996) to habitats similar to Palaearctic populations (running waters in addition to lakes and ponds). In another example, a single specimen of Ametropus fragilis Albarda (Ephemeroptera: Ametropodidae), a rare, large river mayfly that was not even identified to family on original labels, was collected from the Mackenzie River just west of Inuvik. This species is reported to be endangered in all or part of its range (Jacobus Reference Jacobus2013), so may be of conservation interest. Cobb and Flannagan (Reference Cobb and Flannagan1980) reported this species in the Nahanni River in southern Northwest Territories, but this study shows that its distribution extends far to the north in the Mackenzie River Delta. Another rare mayfly, Anepeorus rusticus McDunnough (Ephemeroptera: Heptageniidae) (previously identified as “Rhithrogena sp.” on pipeline study labels), had not been reported in the Northwest Territories before a single specimen was found from the Liard River in the pipeline samples. Although this is an old record, it demonstrates that greater effort should be made to specifically sample for it in the future to determine its distribution. Two fairly common heptageniid mayflies (Heptagenia pulla (Clemens) and Heptagenia solitaria McDunnough) also point to the importance of examining original specimens to assess species patterns. Hepagenia pulla shows two morphological forms in northern Canada, and the one in the study area covered by the pipeline study is the “western form”, in which the asymmetrical mandibles are reversed compared with the eastern form; this may actually be a synonym of the Palaearctic species H. dalecarlica Bengtsson (Jacobus et al. Reference Jacobus, Wiersema and Webb2014). Based on samples examined from across Nunavut and the Northwest Territories (Giberson et al. Reference Giberson, Burian and Shouldice2007 and this study), there does not appear to be an overlap in distribution for the two H. pulla forms, so the eastern and western forms may represent different species. The similar Heptagenia solitaria may also be confused in the west with the eastern form of H. pulla suggesting that the distribution of the two species should be investigated more thoroughly. Cobb and Flannagan (Reference Cobb and Flannagan1980) also noted the presence of Arthroplea bipunctata (McDunnough) (Ephemeroptera: Heptageniidae) and Brachycercus Curtis at one site in the Mackenzie Valley Pipeline study (Martin River; Wiens et al. Reference Wiens, Rosenberg and Snow1975), although these species were not encountered in the re-examination of the samples (possibly due to the specimen not being retained with other archived samples). The report of these species at only one site suggests that targeted sampling may be needed to clarify their complete distribution in the region. For example, A. bipunctata is usually considered to be more of a lentic rather than a lotic species and has not been reported in either Yukon, Canada or Alaska, United States of America. In contrast, Brachycercus has been reported in Alaska to the west (Randolph and McCafferty Reference Randolph and McCafferty2005).
Species diversity comparisons between re-examined (archived) pipeline study specimens and those from more recent collecting confirmed the value of examining archived specimens, even while emphasising the importance of new collecting. Total numbers of species from the two territories was similar among both sets of samples (Table 3), but there were a number of unique species to each set of samples. Since the pipeline samples date from 1970 to 1973, they also provide baseline information for comparing species patterns following nearly half a century of climate change and resource development in northwestern Canada. However, since the sampling locations were not the same, differences may relate to different habitats and locations, as well as surveying intensity. For example, although the “newer” collecting in the Northwest Territories included Mackenzie River tributaries that had been sampled in the pipeline study, it also included northeastern barrenlands sites in and around the Horton and Thelon Rivers, and mountainous streams in the Mackenzie Mountains west of Norman Wells. Recent sampling was also not as long-term or extensive as the original pipeline study, consisting of “spot” sampling, usually at a single sampling time. The more recent sampling missed many species that were captured in the much more extensive and intensive 1970s monitoring study, even as it produced additional species not encountered in the early study. The 50 species identified from the two territories from these samples included additional territorial records (Northwest Territories: four species plus four tentative species; Yukon: one species plus two tentative species [these are in addition to new records from this study already published in Vinke et al. Reference Vinke, Medeiros and Giberson2015; Cordero et al. Reference Cordero, Sánchez-Ramírez and Currie2016]; see Table 3 for list). One notable record is the presence of Baetis vernus Curtis near Yellowknife in Northwest Territories (and Baetis vernus “cf.” in Yukon and the Mackenzie Mountains), since this Palaearctic baetid had not been reported in North America before Cordero et al. (Reference Cordero, Sánchez-Ramírez and Currie2016).
Recent (post-2000) sampling yielded a substantial number of additional records to the Northwest Territories and Yukon lists, despite being far less intensive than the 1970s pipeline study. The high number of additional species relates to sampling additional regions (including the Mackenzie Mountains and central barrenlands), but also because we targeted capture of large, fresh, and intact specimens that are essential for species-level determination, especially when colour patterns are important in identification. Two groups in particular, Siphlonuridae and Ameletidae, are much easier to identify with newer specimens (particularly in concert with new molecular methods of identification), and continued collecting of these groups should help to determine whether the unidentifiable nymphs in this study are of new or known species. We expect that specimens in two heptageniid genera, Rhithrogena and Cinygmula, from both archived and recent samples may yield several additional species if subsequent taxonomic work results in keys to the nymphs of these species.
Overall, these results demonstrate the value of archived collections to generate updated species lists, and to provide information on distributional information and relative abundances. The challenges to working with archived material (especially if label data is not clear), while daunting, can be overcome to provide baseline and distributional data valuable for conservation efforts, and to provide information on where to target further collecting efforts. New collecting is still needed, however, to obtain identifiable material for current distributional information (using both the updated keys and new techniques such as DNA barcoding). Researchers carrying out large ecological or environmental monitoring studies, particularly in isolated or difficult-to-access locations, should archive samples, with clear label data and associated metadata, to be available for future study.
Acknowledgements
The authors thank Dr. David Rosenberg (Emeritus Scientist, Freshwater Institute) for the initial push to examine the Mackenzie River pipeline study specimens for comparison with recent studies along the Mackenzie River. Thanks are also due to Laura Rempel for sharing her Ephemeroptera specimens from the Mackenzie River, and to Sarah Tratch (summer student, University of Prince Edward Island) for her tireless work in deciphering the coded labels for the study. Kristen Vinke and Glen Guthrie collected the mayflies near Norman Wells, and Northern Biodiversity Programme samples were collected by Reuben Cordero, Doug Currie, and their field students.







