Introduction
The Japanese beetle, Popillia japonica Newman (Coleoptera: Scarabaeidae), native to Japan, is a highly polyphagous invasive pest in North America, with both adults and larvae able to cause yield losses and aesthetic damage to ornamental plants and agricultural crops (Shanovich et al. Reference Shanovich, Dean, Koch, Hodgson and Stewart2019; Althoff and Rice Reference Althoff and Rice2022). The Japanese beetle is univoltine in most areas of its native and nonnative geographic ranges, although its lifecycle can take two years to complete in northern areas. Adults mostly emerge in late June or early July and live up to 40 days (Hadley and Hawley Reference Hadley and Hawley1934). Females lay eggs individually in the top 7.5-cm layer of the soil during the summer, with each female laying up to 60 eggs over its lifetime (Dalthorp et al. Reference Dalthorp, Nyrop and Villani2000). The larvae feed on roots and moult until they reach the third instar. Larvae begin overwintering in October after moving down into the soil to a depth of 20–25 cm. In spring, larvae move back up, resume feeding, pupate, and emerge as adults.
The Japanese beetle was first reported in North America in 1916 in New Jersey, United States of America (Clausen et al. Reference Clausen, King and Teranishi1927; Fleming Reference Fleming1976). It has since spread to and established in most of the states east of the Mississippi River, with localised infestations occurring in other states east and west of the Rocky Mountains (United States Department of Agriculture 2023). In Canada, a museum specimen from the Canadian National Collection of Insects, Arachnids and Nematodes (Ottawa, Ontario, Canada) was traced back the first detection of the Japanese beetle in 1929 in southern Ontario (Gagnon and Giroux Reference Gagnon and Giroux2019). In Québec, individuals were first trapped in 1939 in Lacolle (Garland Reference Garland1990). Populations are now established throughout eastern Canada, from Ontario eastwards, with the exception of Newfoundland (Canadian Food Inspection Agency 2023).
More than a century ago, the solitary, adult parasitoid Istocheta aldrichi (Mesnil) (Diptera: Tachinidae), native to the eastern Palearctic region (Herting and Dely-Draskovits Reference Herting and Dely-Draskovits1993), was selected for releases in the United States of America to control Japanese beetle populations (Clausen et al. Reference Clausen, King and Teranishi1927). Following repeated introductions from 1920 to 1950, I. aldrichi spread to and established in New York, Pennsylvania, New Jersey, Massachusetts, Connecticut, and the District of Columbia (O’Hara and Wood Reference O’Hara and Wood2004) and more recently in Minnesota and North Carolina (Klein and McDonald Reference Klein and McDonald2007; Shanovich et al. Reference Shanovich, Ribeiro and Koch2021). It is assumed that Canadian I. aldrichi populations spread from its introduced range in the United States of America. Parasitised Japanese beetles were first observed in Granby, Québec in 2009 (Gagnon and Giroux Reference Gagnon and Giroux2019) and in the Ottawa region of Ontario in 2013 (O’Hara Reference O’Hara2014). Surveys of entomological collections and community science databases (http://www.inaturalist.org) further provided evidence that I. aldrichi was present in several locations of southern Québec as of 2017 (Gagnon and Giroux Reference Gagnon and Giroux2019).
Most of the information on the biology and natural history of I. aldrichi come from Clausen et al.’s (Reference Clausen, King and Teranishi1927) landmark study: the female parasitoid lays its eggs on the pronotum of its host. Upon eclosion, the fly larva drills downwards into the beetle and starts feeding on host tissues. Parasitised P. japonica next burrow into the soil and die. The fly larva pupates within the host cadaver and overwinters. Istocheta aldrichi is univoltine (Clausen et al. Reference Clausen, King and Teranishi1927), and the parasitoid appears to be specific to P. japonica (Arnaud Reference Arnaud1978; O’Hara and Wood Reference O’Hara and Wood2004), although rigorous host specificity testing has not been conducted.
The present study investigated the distribution, seasonal occurrence, and parasitism rates of I. aldrichi in Québec following its initial discovery. To accomplish this, we used baited traps in 2018 and 2019 to collect parasitised beetles in eight localities of southern Québec. In 2022, we recorded parasitism rates in 13 raspberry (Rubus idaeus) fields localised along a latitudinal gradient. Finally, we used data from the online platform iNaturalist to further complement the current distribution of I. aldrichi. This study is part of a larger research program designed to first monitor the spread, establishment, and dispersal of I. aldrichi in Canada and then to evaluate the population-level impact of I. aldrichi on P. japonica in invaded areas.
Materials and methods
Parasitism in southern Québec
In 2018 and 2019, parasitism of P. japonica by I. aldrichi was monitored from July to October at eight sampling sites where Japanese beetle populations were presumably established based on information from agronomists and iNaturalist observations (Fig. 1A; Table 1). Parasitism was estimated using Bioprotec® traps with a dual lure system comprised of a floral bait (phenethyl propionate + eugenol + geraniol (3:7:3); Ladd and Klein Reference Ladd and Klein1986) and the synthetic sex pheromone of the Japanese beetle, “Japonilure” ((R,Z)-5-(1-decencyl)-dihydro-2(3H)-furanone; Tumlinson et al. Reference Tumlinson, Klein, Doolittle, Ladd and Proveaux1977). These traps provide reliable estimates of parasitism rates (Legault et al. Reference Legault, Doyon and Brodeur2023).
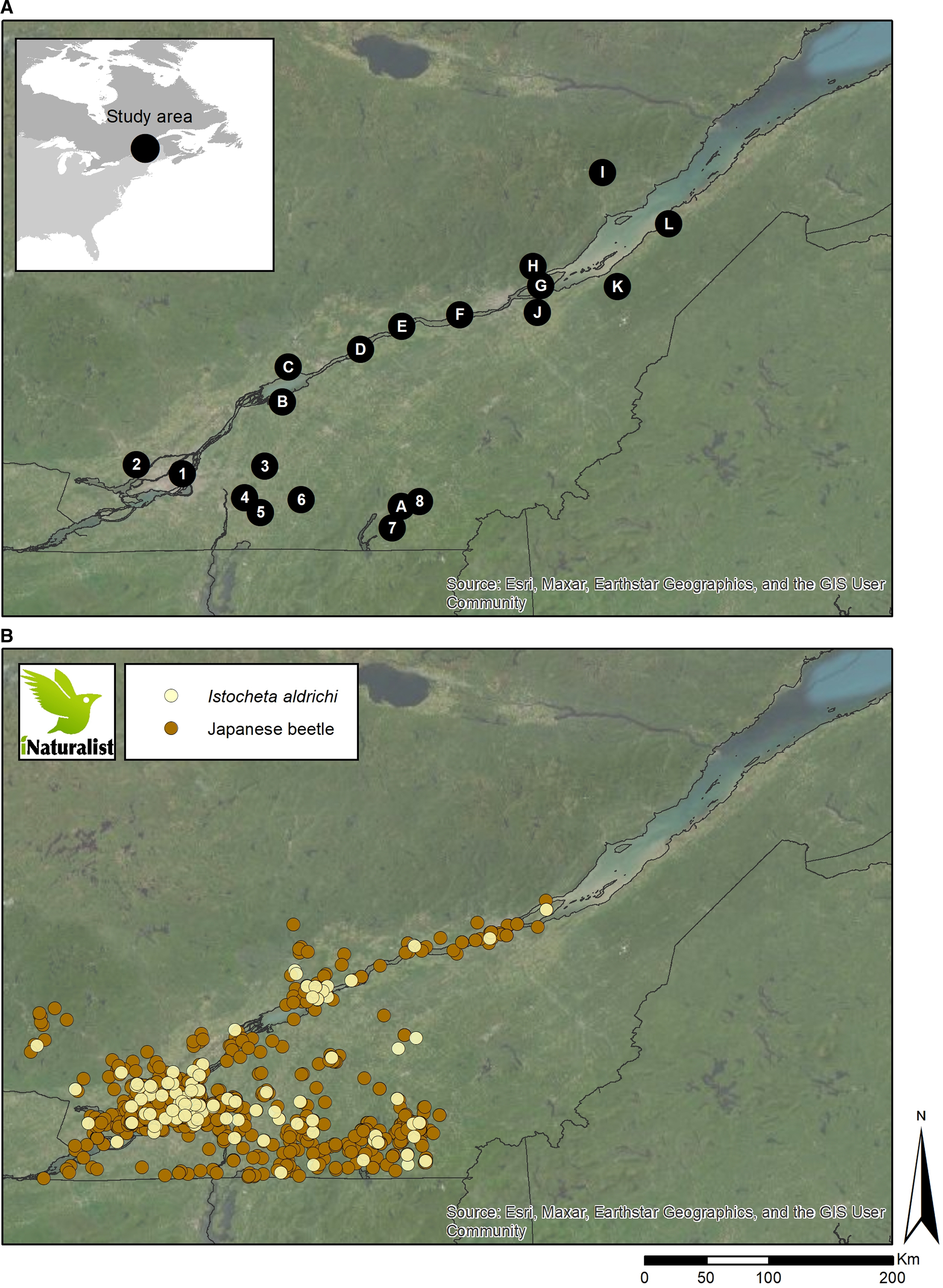
Figure 1. A, Sampling locations in southern Québec in 2018–2019 (1–8) and 2022 (1; A–L). B, Spatial distribution of the Japanese beetle (orange dots) and Istocheta aldrichi (yellow dots) in Québec as of 2022, based on iNaturalist.
Table 1. Parasitism of Japanese beetles’ adults by Istocheta aldrichi in eight sampling locations in southern Québec, Canada, in 2018–2019. Site numbers correspond to Figs. 1A and 2. For each sampling period, %Em corresponds to the estimated cumulative emergence of Japanese beetles based on the degree-day model of Ebbenga et al. (Reference Ebbenga, Hanson, Burkness and Hutchison2022; see Methods section for details). Max I.a. – maximum parasitism rate by I. aldrichi; Date Max – date for Max I.a. estimation, Adj. Tot I.a. – total parasitism rate by I. aldrichi over the season, adjusted for missing Japanese beetle collections based on the degree-day model of Ebbenga et al. (Reference Ebbenga, Hanson, Burkness and Hutchison2022); N – number of Japanese beetles examined for parasitism estimation.

‡ Date Max corresponds to earliest captures of Japanese beetles.
Traps were positioned in or nearby botanical gardens, plant nurseries, resident gardens, or experimental farms (Table 1). Each sampling site was characterised by a relatively diverse plant community. Within each sampling site, one trap was placed on a U-shaped bamboo pole 1 m above the ground. In 2018 and 2019, traps were deployed no later than 4 July and 11 July, respectively (Table 1), when Japanese beetles became active in the field, based on information from collaborators. Sampling ended in late August–early September in 2018 and late September–early October in 2019, when Japanese beetle populations were declining (Table 1). The same lures were used because their attractiveness does not decrease throughout the season (Ladd et al. Reference Ladd, Klein, Lawrence and Beroza1974). Traps were emptied once a week, and beetles were sent to the Institut de Recherche en Biologie Végétale (Montréal, Québec, Canada) and frozen at –20 °C in plastic bags until processing. For each sample, all beetles were counted and examined under a stereomicroscope for the presence of I. aldrichi eggs to estimate weekly and total seasonal parasitism rates (i.e., number of beetles bearing at least one parasitoid egg/total number of beetles × 100%).
For each location, total parasitism rates over the course of the season were calculated based on the total number of Japanese beetles trapped. Because we suspected that we missed some beetles in early and late season, we used the degree-day model of Ebbenga et al. (Reference Ebbenga, Hanson, Burkness and Hutchison2022) to estimate the proportion of unsampled Japanese beetles. For each location, we used the weather generator of BioSIM 11 (cfs.nrcan.gc.ca/projects/133) to estimate daily minimum and maximum air temperatures. Temperatures for each location were estimated through distance-weighted interpolation using up to eight weather stations nearest to each of the sampling locations (Régnière and St-Amant Reference Régnière and St. Amant2007). For each sampling location and year, simple degree-day accumulations were calculated using a biofix date of 1 January and lower and upper thresholds of 15.0 and 21.7 °C, respectively (Ebbenga et al. Reference Ebbenga, Hanson, Burkness and Hutchison2022). Accumulated degree days for each sampling date were then converted to the expected proportion of emerged Japanese beetles using Ebbenga et al.’s (Reference Ebbenga, Hanson, Burkness and Hutchison2022) equation (1). These proportions of expected unsampled Japanese beetle captures were then used to adjust total parasitism rates by I. aldrichi throughout the season.
Parasitism along the St. Lawrence River
In 2022, parasitism of P. japonica by I. aldrichi was monitored during the seasonal peak activity of I. aldrichi, from early July to early August, at 13 sampling sites along the St. Lawrence River (latitudinal gradient; Fig. 1A; Table 2). Insects were sampled on raspberry farms, except for at Site 1 (Montréal Botanical Garden) and Site A, which consisted of an open area with wild raspberry bushes, raspberry plants being commonly exploited by Japanese beetles North America (Burkness et al. Reference Burkness, Ebbenga, Toninato and Hutchison2022). At each location, one Bioprotec® trap was positioned near raspberries on a wooden pole 1 m above the ground. Traps were emptied twice during the sampling period (Table 2). All the samples were processed as described for previous years.
Table 2. Parasitism of Japanese beetle adults by Istocheta aldrichi in 13 sampling locations along the presumed expansion front of I. aldrichi in Québec, Canada, in 2022. Site numbers correspond to Fig. 1A. For each site and sampling period, P I.a. is the parasitism rate by I. aldrichi over the period, and N is number of Japanese beetles examined for parasitism estimations.

iNaturalist observations
iNaturalist (http://www.inaturalist.org) is an open-access platform where anyone, including naturalists and scientists, can share their observations of biodiversity. We accessed the database on 3 April 2023 to document the occurrence and distribution of naturalised Japanese beetle and its parasitoid, I. aldrichi, in the province of Québec using the following search terms: “Popillia japonica” and “Istocheta aldrichi”. We downloaded and mapped the geographic coordinates of all “Research Grade” observations from iNaturalist using the package rinat (Barve and Hart Reference Barve and Hart2022) for R (R Core Team; https://www.R-project.org).
Results
Parasitism in southern Québec
A total of 24 807 and 26 667 adult Japanese beetles were captured in baited traps in 2018 and 2019, respectively. Total beetle abundances among site–years are shown in Table 1. Japanese beetles first appeared in traps in late June–early July, and population densities generally increased rapidly for several weeks before declining more gradually (Fig. 2). Overall, seasonal abundances were more or less unimodal, with maxima occurring from mid-July to early August.

Figure 2. Seasonal distribution of the relative (%) abundance of Popillia japonica (![]() ) and percent parasitism by Istocheta aldrichi (
) and percent parasitism by Istocheta aldrichi (![]() ) in eight locations in southern Québec, Canada, in 2018–2019. Site numbers are as in Table 1 and Fig. 1A. Records with low sample sizes (1 < N > 30) are indicated with a
) in eight locations in southern Québec, Canada, in 2018–2019. Site numbers are as in Table 1 and Fig. 1A. Records with low sample sizes (1 < N > 30) are indicated with a![]() symbol. Records with no Japanese beetles are indicated with a
symbol. Records with no Japanese beetles are indicated with a![]() symbol.
symbol.
In 2018 and 2019, parasitised Japanese beetles were found at all sites. The appearance of I. aldrichi eggs on its host in late June coincided with the emergence of the first Japanese beetles. In all sites and for both years, with the exception of Sherbrooke, maximum parasitism (Table 1) occurred during the first two weeks following Japanese beetle emergence before declining gradually. Parasitoid eggs were rarely observed after mid-August (Fig. 2). Over all sites and sampling dates, maximum parasitism levels ranged from 14.1 to 50%, with the caveat of small sample size (N < 30) in most sites early in the season (Table 1; Fig. 2). Total parasitism rates over the course of the two years ranged from 3.9% in Boisbriand in 2019 to 27.3% in Ayer’s Cliff in 2018 (Table 1).
Parasitism along the St. Lawrence River
In 2022, surveys indicated that both the Japanese beetle and its parasitoid were present in raspberry fields from Montréal (southwest) to Neuville (northeast; Table 2). However, a single unparasitised beetle was trapped in Beaumont (site K), 60 km east of the current front of expansion of the Japanese beetle along the St. Lawrence River (Table 2). Parasitism rates over the sampling period ranged from 4.6 to 23.7%.
iNaturalist observations
The iNaturalist website reported a total of 1792 and 167 occurrences of Japanese beetles and I. aldrichi, respectively, between 2003 and 2022 in the province of Québec. The first observations of the Japanese beetle and I. aldrichi were in 2003 and 2014, respectively. These observations, presented in Fig. 1B, indicate that, as of 2022, the Japanese beetle was present throughout southern Québec and along the St. Lawrence River, up to Ile d’Orléans (46.8306, –70.9934), north of Quebec City. The reported observations of I. aldrichi in iNaturalist are spatially similar to that of the Japanese beetle within the province.
Discussion
This study aimed to explore ecological aspects of the early establishment in Québec, Canada, of the association between two exotic and now naturalised insects: the Japanese beetle, P. japonica, and the tachinid parasitoid, I. aldrichi. Our results indicate that (1) I. aldrichi is well spread in most areas where the Japanese beetle is present in southern Québec, (2) parasitism mostly occurs from late June to mid-July, before the peak emergence of Japanese beetle populations, and (3) levels of total seasonal parasitism ranged from 3.9 to 27.3% across sampled sites in 2018, 2019, and 2022.
The surveys conducted in eight locations in 2018 and 2019 and 13 in 2022, combined with the information retrieved from iNaturalist, show that the Japanese beetle and its parasitoid are widespread in Québec. There is no comprehensive record of the spread and invasion routes of the Japanese beetle in Canada. Data from the Canadian Food Inspection Agency indicate that in 1996, populations of the Japanese beetle were restricted to five regulated areas in southern Québec within the boundaries of the regional county municipalities (MRC) of Brome-Missisquoi, Le Haut-Richelieu, Champlain, Roussillon, and Le Bas-Richelieu, with no observations on the north shore of the St. Lawrence River and northeast of the area of Lac St. Pierre (Canadian Food Inspection Agency 1996). In 2005, the beetle had expanded its distribution to 28 regulated areas, up to the Centre-du-Québec region (Bois-Francs; Canadian Food Inspection Agency 2005). In 2006, the Canadian Food Inspection Agency stopped monitoring the Japanese beetle in Québec (Canadian Food Inspection 2006). Our survey and the information retrieved from iNaturalist constitute the most updated observations on the current distribution of the Japanese beetle. In Québec, I. aldrichi was first detected (a single mention) in Granby (see location on Fig. 1) in 2009, and the present study indicates that it rapidly spread through other parts of the province where the Japanese beetle is present.
In Japan, the parasitoid–host’s life cycle is well synchronised, and parasitism begins upon the spring emergence of the first beetles and extends through most of the period of host activity (King Reference King1931). Our trap captures in 2018–2019, however, suggest a mismatch in the spring emergence of diapausing Japanese beetles and I. aldrichi in Québec. This results in early emerging beetles experiencing the highest levels of parasitism, followed by a long period of less vulnerability to parasitism when beetle populations are peaking. Such a pattern has been observed in several areas in North America following the repeated introductions of I. aldrichi in 1920–1950 (King Reference King1931; Fleming Reference Fleming1968; Shanovich et al. Reference Shanovich, Dean, Koch, Hodgson and Stewart2019). Postrelease monitoring revealed that I. aldrichi emerges approximately 2–3 weeks before the peak of its host’s emergence (King Reference King1931). This host–parasitoid phenological asynchrony could greatly reduce the effectiveness of I. aldrichi as a biological control agent of the Japanese beetle.
Clausen et al. (Reference Clausen, King and Teranishi1927) and King (Reference King1931) concluded that P. japonica is of minor economic importance in its native range in Japan. This was attributed to biological control exerted by multiple natural enemies, the most effective of which was thought to be I. aldrichi (Clausen et al. Reference Clausen, King and Teranishi1927). Those authors reported total seasonal parasitism of Japanese beetles by I. aldrichi fluctuating between 20 and 90% in Hokkaido and 50% on the island of Honshu. In North America, total seasonal parasitism has rarely been quantified. To our knowledge, the only published records come from the studies of Shanovich et al. (Reference Shanovich, Dean, Koch, Hodgson and Stewart2019, Reference Shanovich, Ribeiro and Koch2021 in Minnesota, where approximately 10 and 9.3% parasitism was observed in urban areas and apple orchards, respectively. Our sampled sites in Québec were distributed widely across the province, covering an estimate of 35 000 km2, and total seasonal parasitism varied from 3.9 to 27.3% across sites and years.
Our results provide an overview of the early establishment of the relationship between the Japanese beetle and I. aldrichi in Québec. Surprisingly, despite the pest status of the Japanese beetle and given the repeated efforts to release and redistribute I. aldrichi in North America, postrelease data are insufficient to understand several important aspects of their ecology, such as their phenology, response to climatic conditions, and seasonal population dynamics. This information is essential not only to prevent further Japanese beetle introductions but also to maximise the management of established pest populations through a better understanding of the impact of naturalised I. aldrichi populations within the continent. Ongoing research in Canada aims at providing quantitative analyses of the seasonal population dynamics of the Japanese beetle–I. aldrichi association, the continuous spread of both insects, the effectiveness of I. aldrichi as a biological control agent, and its redistribution in new invaded areas, as well as information on the biology of I. aldrichi to complement the pioneering work conducted a century ago by Drs. Clausen, King, and Teranishi.
Acknowledgements
The authors sincerely thank the Institut Québécois du Développement de l’Horticulture Ornementale (IQDHO) for their contributions for monitoring insect populations, the Montréal Botanical Garden, and the raspberry growers for allowing us to use their fields. They thank Sean-Anthony Di-Paolo and Julie-Anne Pelland for technical assistance. They are also grateful to reviewers for their constructive suggestions that greatly improved the quality of the manuscript. This work was supported by the Insectarium de Montréal and the Canada research chair in biological control to J.B.
Competing interests
The authors declare they have no competing interests.







