Introduction
The taxonomy of the subfamily Scolytinae has traditionally been based on external morphological attributes that correspond to the elytral declivity, head, and pronotum because their variation allows species identification and because these body parts display cuticle elements easily observable and quantifiable in terms of abundance, size, and distribution (Hopkins Reference Hopkins1909; Wood Reference Wood1982; Hulcr et al. Reference Hulcr, Atkinson, Cognato, Jordal, McKenna, Vega and Hofstetter2015).
In insects, particularly in Coleoptera, male genital attributes are important for taxonomy (Coca-Abia and Robbins Reference Coca-Abia and Robbins2006; Pardo-Diaz et al. Reference Pardo-Diaz, Toro, Tovar, Sarmiento-Garcés, Herrera and Salazar2019; Yang et al. Reference Yang, Yang and Shi2020; Gao and Coca-Abia Reference Gao and Coca-Abia2021); however, these attributes have been poorly integrated in bark beetle studies due to the difficulty of manipulating small specimens and structures (Hulcr et al. Reference Hulcr, Atkinson, Cognato, Jordal, McKenna, Vega and Hofstetter2015). Although the genitalia have been explored in Scolytinae for taxonomic purposes (Wood Reference Wood1957; Hopping Reference Hopping1963; Lanier Reference Lanier1972, Reference Lanier1987; Pajares and Lanier Reference Pajares and Lanier1990; Lanier et al. Reference Lanier, Teale and Pajares1991; Furniss Reference Furniss1996; Mandelshtam et al. Reference Mandelshtam, Petrov, Axentjev and Knížek2006, Reference Mandelshtam, Petrov and Korotyaev2012), detailed and focused study of the morphology of the endophallus has not been carried out to date. In a few taxa, specialised parts of male genitalia can provide good taxonomic traits. These parts include the aedeagus, seminal rod, and anchor shape in Camptocerus Dejean (Smith and Cognato Reference Smith and Cognato2010), Carphobious Blackman (Cognato and Smith Reference Cognato and Smith2023), members of the Cryphalini (Johnson et al. Reference Johnson, Hulcr, Knížek, Atkinson, Mandelshtam and Smith2020; Justesen et al. Reference Justesen, Hansen, Knížek, Lindelow, Solodovnikov and Ravn2023), Dendroctonus Erichson (Rios-Reyes et al. Reference Rios-Reyes, Valdez-Carrasco, Equihua-Martínez and Moya-Raygoza2008; Armendáriz-Toledano et al. Reference Armendáriz-Toledano, Niño, Macías Sámano and Zúñiga2014; García-Román et al. Reference García-Román, Armendáriz-Toledano, Valerio-Mendoza and Zúñiga2019, Reference García-Román, Ramírez-Reyes and Armendáriz-Toledano2022; Valerio-Mendoza et al. Reference Valerio-Mendoza, García-Román, Becerril, Armendáriz-Toledano, Cuéllar-Rodríguez and Negrón2019), the Hylastini (Mandelshtam and Petrov Reference Mandelshtam and Petrov2019), the Hypoborini (Jordal Reference Jordal2021b), the Micradicini (Jordal Reference Jordal2021a), and Xyloctonus Erichhoff (Jordal Reference Jordal2024), and the receptacle in Xyleborus Eichhoff (Pérez-Silva et al. Reference Pérez-Silva, Equihua Martínez and Atkinson2015), whereas in other beetles, such as Scolytus Geoffroy, male genitalia display extremely conserved patterns (Johnson et al. Reference Johnson, Hayes, Rinehart, Sheppard and Smith2008; Smith and Cognato Reference Smith and Cognato2014).
A little-explored structure with taxonomic potential is the endophallus, an eversible membranous sac located within the aedeagus (Tuxen Reference Tuxen1970; Nichols Reference Nichols1989). During the copulation process, the endophallus is placed inside the bursa copulatrix, functioning as a lock-and-key system and as a prezygotic isolation mechanism (Düngelhoef and Schmitt Reference Düngelhoef and Schmitt2010), making it useful for defining species boundaries and estimating phylogenetic relationships (Coca-Abia Reference Coca-Abia2007; Düngelhoef and Schmitt Reference Düngelhoef and Schmitt2010; Sasabe et al. Reference Sasabe, Takami and Sota2010; Erbey and Candan Reference Erbey and Candan2018). In several Coleoptera taxa, the endophallus is a powerful taxonomic tool for delimiting and identifying problematic species (Danilevsky and Kasatkin Reference Danilevsky and Kasatkin2004; Bollino and Sandel Reference Bollino and Sandel2017).
The first comparative study of the anatomy of the male genitalia in the order Coleoptera corresponds to Sharp and Muir (Reference Sharp and Muir1912). They described characteristics of the shape and structures of the endophallus, incorporated a technique for its eversion, and emphasised the importance of including it in taxonomic studies. Since this work was published, the endophallus morphology has contributed significantly to the understanding of the taxonomy of different beetle families, such as Lucanidae (Imura Reference Imura2007), Glaphyridae (Uliana and Sabatinelli Reference Uliana and Sabatinelli2010; Bollino and Ruzzante Reference Bollino and Ruzzante2015), Chrysomelidae (Bukejs and Anichtchenko Reference Bukejs and Anichtchenko2019; Daccordi et al. Reference Daccordi, Bollino and Vela2020), Carabidae (Anichtchenko Reference Anichtchenko2010; Janovska et al. Reference Janovska, Anichtchenko and Erwin2013), Curculionidae (Bollino and Sandel Reference Bollino and Sandel2017; Meregalli et al. Reference Meregalli, Boriani, Taddei, Hsu, Tseng and Mouttet2020), and Cerambycidae (Danilevsky and Kasatkin Reference Danilevsky and Kasatkin2004; Yamasako and Ohbayashi Reference Yamasako and Ohbayashi2011), among others.
In some taxa of Scarabaeoidea, the everted and noninflated endophallus’s structural characteristics, such as sclerites, spines, and silks, have been used as attributes for phylogenetic inferences (Coca-Abia Reference Coca-Abia2007). In Scolytinae, the endophallus has been studied only in Dendroctonus monticolae Hopkins (= D. ponderosae) (Cerezke Reference Cerezke1964): that study focused on describing the inflated endophallus and the musculature associated with its eversion movement but did not present the technique used for inflation.
The diversity of the Coleoptera groups in which the endophallus has been studied allowed the development of several inflation techniques using different substances to inflate and fill this structure. The Berti–Vachon technique, or “air filling” (Bontems Reference Bontems2013), is one of the most commonly used because it recovers the endophallus shape and maintains the integrity of the structures of the internal sac; however, its execution is complicated, especially in small specimens. Another commonly used technique is that of Berlov (Reference Berlov1992), which consists of filling the sac with toothpaste. Although this method recovers the endophallus shape, details of the sclerotised structures are lost because of the toothpaste’s colour and the internal sac loses volume during the drying process. To avoid this problem, Uliana and Sabatinelli (Reference Uliana and Sabatinelli2010) proposed a modification to Berlov’s technique that adds micronised silica to reduce these effects. Yamasako and Ohbayashi (Reference Yamasako and Ohbayashi2011) showed that using glycerin as a filling substance is also effective; the only disadvantage is that the structure cannot be preserved dry as it can with the previous techniques. Van Dam (Reference Van Dam2014) developed a new inflation technique for small curculionids, applicable to both fresh and museum specimens; this method consists of cleaning the aedeagus with an enzymatic solution and then using K-Y gel to fill the endophallus. Although Van Dam’s (Reference Van Dam2014) technique yields effective results and maintains the transparency of the membrane, the sample also cannot be kept dry.
In this study, we propose modifications to Van Dam’s (Reference Van Dam2014) technique for the study of the endophallus of Scolytinae members. In addition, we describe the anatomy and morphology of the internal sac for the first time in 16 species from Dendroctonus Erichson, Ips DeGeer, and Phloeosinus Chapuis, emphasising the sac’s usefulness in taxonomy. We also describe and discuss the inflation patterns of the internal sac.
Materials and methods
Sixty specimens corresponding to 16 species of three genera of Scolytinae were analysed. Of these, 10 were Dendroctonus Erichson spp.: D. adjunctus Blandford, D. approximatus Dietz, D. barberi Hopkins, D. frontalis Zimmerman, D. mesoamericanus Armedáriz-Toledano and Sullivan, D. parallelocollis Chapuis, D. pseudotsugae barragani Furniss, D. vitei Wood, D. rhizophagus Thomas and Bright, and D. valens (LeConte). Four were Phloeosinus Chapuis spp. (Coleoptera: Curculionidae): P. baumanni Hopkins, P. deleoni Blackman, P. tacubayae Hopkins, and P. serratus LeConte. Two were Ips DeGeer spp. (Coleoptera: Curculionidae): I. lecontei Swaine and I. calligraphus (Germar) (Table 1). The specimens examined included those obtained from infested trees in 21 localities in Mexico and Honduras, as well as museum specimens, some of which were mounted and some of which were preserved in alcohol. Museum specimens were borrowed from the Colección Nacional de Insectos, Instituto de Biología, Universidad Nacional Autónoma de México, Mexico, and the collected specimens were deposited in it. Specimens from different populations were included in D. valens and P. serratus because these species are known for their cryptic diversity (Ramírez-Reyes et al. Reference Ramírez-Reyes, Armendáriz-Toledano and Rodríguez2023).
Table 1. Locations, geographical coordinates, hosts of examined Dendroctonus, Ips, and Phloeosinus specimens, and the collection where they are found. Specimens supporting these records were deposited in the Colección Nacional de Insectos, Instituto de Biología, Universidad Nacional Autónoma de México

To sex and identify specimens, we used dichotomous keys. Dendroctonus spp. were sexed based on external body traits and the shape of the seventh tergite (Armendáriz-Toledano et al. Reference Armendáriz-Toledano, Niño, Sullivan, Kirkendall and Zúñiga2015) and by the presence of stridulatory apparatus in males (Lyon Reference Lyon1958). Species identification was made based on external and internal morphology attributes, such as the presence or absence of frontal tubercules, the length and abundance of pubescence of elytral declivity (in D. adjunctus and D. approximatus; García-Román et al. Reference García-Román, Armendáriz-Toledano, Valerio-Mendoza and Zúñiga2019), the presence of coarse rugosities on the elytral interspaces and impressed striae, and the short pubescence on the elytral declivity (in D. barberi; Valerio-Mendoza et al. Reference Valerio-Mendoza, García-Román, Becerril, Armendáriz-Toledano, Cuéllar-Rodríguez and Negrón2019), the shape of the lateral margins of the epistomal process and pronotum (in D. parallelocollis and D. pseudotsugae barragani; Armendáriz-Toledano and Zúñiga Reference Armendariz-Toledano and Zúñiga2017; García-Román et al. Reference García-Román, Armendáriz-Toledano, Valerio-Mendoza and Zúñiga2019), characteristics of the antennal club (in D. rhizophagus, D. valens, and D. vitei; Armendáriz-Toledano et al. Reference Armendáriz-Toledano, Niño, Macías Sámano and Zúñiga2014), and characteristics of the male genitalia such as the seminal rod and anchor (in D. frontalis and D. mesoamericanus; Armendáriz-Toledano et al. Reference Armendáriz-Toledano, Niño, Macías Sámano and Zúñiga2014, Reference Armendáriz-Toledano, Niño, Sullivan, Kirkendall and Zúñiga2015).
Phloeosinus specimens were sexed based on whether the eighth tergite was visible (males) or not (females; Hopkins Reference Hopkins1905; Cervantes-Espinoza et al. Reference Cervantes-Espinoza, Ruiz, Cuellar-Rodríguez, Castro-Valderrama and Armendáriz-Toledano2023). The species were identified based on external morphological characters (Blackman Reference Blackman1942; Wood Reference Wood1982). Characters from the head were used, including frons shape, the elevation of the carina concerning the epistomal margin, and the shape of antennal sutures, the density of pubescence and punctuations of pronotum, the shape of elytral declivity, the width of interstriae, and the number and shape of the declivital teeth (in P. baumanni, P. deleoni, P. serratus, and P. tacubayae)
Finally, the Ips specimens were identified using the dichotomous keys of Lanier (Reference Lanier1987), Douglas et al. (Reference Douglas, Cognato, Grebennikov and Savard2019), and LaBonte and Valley (Reference LaBonte and Valley2019). The I. calligraphus specimens were sexed based on the hook shape of the third spine of the elytral declivity, whereas I. lecontei males were identified based on the presence of bifurcate tubercles at the centre of the epistomal margin.
Male adults were dissected to obtain the genitalia. The methods used to obtain the aedeagus differed according to how the specimens were preserved (dry, alcohol, or fresh) and are described below.
Rehydration of dry-mounted specimens. For optimal extraction of the genitalia, the specimens were first rehydrated using a solution of distilled water and 50% commercial meat tenderiser to soften them. The ingredients of the meat tenderiser, as indicated on the product label, were iodised salt, dextrose, papain, and calcium stearate. The procedure consisted of placing the insects in vials with this solution and incubating them for 45–60 minutes at 60 °C.
Obtaining and cleaning the aedeagus. After rehydration, the dissection to obtain the aedeagus was performed for all specimens (preserved dry, preserved in alcohol, and fresh). The abdomen was removed and placed in a vial with a pancreatin solution to facilitate tissue digestion (Álvarez-Padilla and Hormiga Reference Álvarez-Padilla and Hormiga2007; Van Dam Reference Van Dam2014); the samples were incubated for 1–2 hours at 37 °C, although they can also be left at room temperature for 2–3 days. Once the samples were clean, they were rinsed with distilled water and then preserved in alcohol. If the tissue did not dissolve completely, the samples were incubated for 30 minutes at 60 °C with 10% NaOH to finish cleaning and softening, then they were neutralised with 10% HCl, rinsed with distilled water, and preserved in 70% alcohol.
Endophallus inflation . Once the aedeaguses were clean, the endophalluses were inflated. Van Dam’s (Reference Van Dam2014) technique was used, with modifications. An insulin syringe was used to inflate each endophallus: the tip of the needle was filed to a right angle, and the contour was filed to obtain a conical shape and to facilitate inflation. A lubricant K-Y gel was used as an inflation substance. Because of the gel’s density, it was diluted to 50% dilution with 96% alcohol before inflation.
A drop of the gel solution was placed on a slide, and an aedeagus was placed in it. The procedure was performed using a stereo microscope. With the help of fine-tipped forceps, the body of the aedeagus was held, taking care not to crush it, and it was placed in a dorsal position or in a position that allowed easy manipulation for inflation. With the syringe, the gel was injected through the basal orifice of the aedeagus located between the apodemes (Fig. 1). Once the endophallus was everted, it was photographed from various aspects – dorsal, lateral, and ventral – for subsequent description. A compound microscope with a camera at 40×, 100×, and 400× magnification, depending on species size, was used to photograph the endophallus. Finally, the samples were preserved in a vial with 70% alcohol. For some species, the inflation process was video-recorded to capture the movement of the structures involved; however, in the case of the genus Ips, this was the only option to describe it due to the intrinsic characteristics of the endophallus (see Results). From the photographs and videos, drawings of the different views of the endophallus of all species were made.
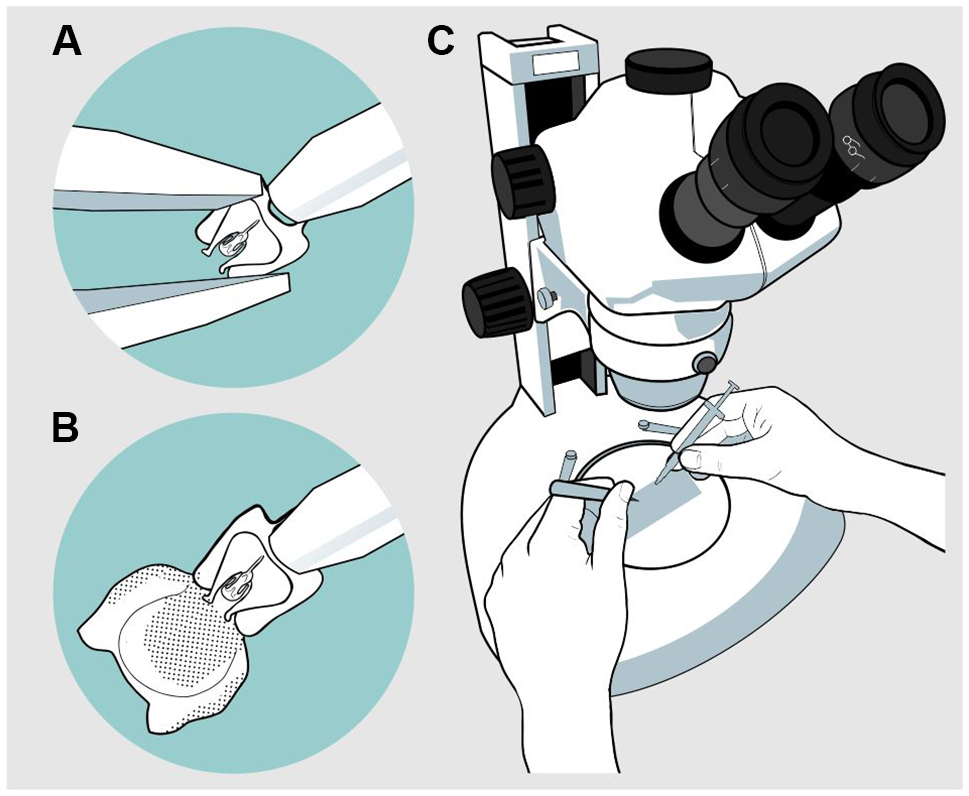
Figure 1. Endophallus inflation process: A, first, the forceps are fixed around the base of the basal orifice with the blunt-tipped syringe in the centre; B, then the endophallus is inflated; and C, the process is realised with a slide and syringe under stereoscopic microscope.
The endophallus anatomy was described using the nomenclature of Cerezke (Reference Cerezke1964), Tuxen (Reference Tuxen1970), Nichols (Reference Nichols1989), Yamasako and Ohbayashi (Reference Yamasako and Ohbayashi2011), Daccordi et al. (Reference Daccordi, Bollino and Vela2020), and Meregalli et al. (Reference Meregalli, Boriani, Taddei, Hsu, Tseng and Mouttet2020). The definitions for describing the structures of the aedeagus and endophallus were based on the glossary of Cerezke (Reference Cerezke1964), Tuxen (Reference Tuxen1970), Nichols (Reference Nichols1989), and Armendáriz-Toledano et al. (Reference Armendáriz-Toledano, Niño, Sullivan, Kirkendall and Zúñiga2015). Use of the terms “aedeagus” and “penis” is restricted in the present study to the body part commonly known as the median lobe, which contains the accessory apparatus and the internal sac, and does not include the spicule and tegmen. A list of abbreviations of aedeagus terminology to describe endophallus morphology is presented in Supplementary material, file S1.
Results
Inflation
Of the 16 species analysed, two were only partially inflated because they were dry-preserved specimens with very small aedeaguses. In the Dendroctonus and Phloeosinus specimens, the inflated endophallus was maintained for days after the procedure, which facilitated their observation and description. In the Ips specimens, the endophallus maintained its shape only when the pressure of the injection fluid was constant: when the fluid was no longer applied, the sac retracted into the aedeagus, suggesting a stronger retraction mechanism (Supplementary material, video S1).
Morphology
Our results show that, in Dendroctonus, Phloeosinus, and Ips, the genital organ consists of three sclerotised structures – the spicule, tegmen, and penis (sensu Cerezke Reference Cerezke1964) or aedeagus (sensu Nichols Reference Nichols1989). The spicule and tegmen lie adjacent to the penis but are attached ventrally to it by muscle and membranes. The tegmen is a small transversal segment, irregular in shape, that is attached to apodemes; the structure provides musculature support during eversion (Cerezke Reference Cerezke1964). In Dendroctonus, the tegmen is a U-shaped structure located in the ventral region of the aedeagus close to the apodemes, whereas in Phloeosinus, it is V-shaped, and in Ips, it is a ring surrounding the aedeagus. The spicule is a small, parallel, needle-like spine attached by membranes to the outside of the aedeagus (Tuxen Reference Tuxen1970); distally, the spine is bifurcated. Partially surrounding the aedeagus, the spicule supports the aedeagus. The spicule is similar in shape among all species, differing between the species primarily in size.
In the Dendroctonus and Phloeosinus spp., we found that the endophallus and an accessory apparatus are present within the aedeagus, the accessory apparatus consisting of the seminal rod and an anchor.
The aedeagus is a capsule with two orifices, the basal one in the proximal region and the apical one, or ostium, in the distal region. In the basal region are the apodemes, which correspond to a pair of thin extensions that support the spermatic duct; the length of apodemes varies among genera and species, mainly among Ips. The seminal rod and anchor conform to the accessory apparatus in Dendroctonus and Phloeosinus: these structures vary within species, mainly among Dendroctonus. Both structures are involved in the endophallus eversion process during copulation.
The endophallus in the taxa examined in the present study is a sac that is continuously connected to the distal end of the aedeagus. When the endophallus is fully everted and inflated, it looks like a semitransparent membrane that is composed of lobes (Fig. 2). During the eversion process, the endophallus exits through the ostium in Dendroctonus, driven by the movement of the accessory apparatus to which it is attached (Fig. 2A). In Phloeosinus, the endophallus ascends through the dorsal–distal region of the aedeagus, where it joins at the lateral folds, and in the dorsal–proximal region, it joins with the accessory apparatus (Fig. 2B). In both Dendroctonus and Phloeosinus, when the endophallus is everted, the accessory apparatus is displaced at an angle of 45–90° from its uninflated position. In Ips, the endophallus is attached dorsally to the median lobe of the aedeagus and laterally to the ventral lobe, with both the median and ventral lobes limiting the movement of the endophallus during the eversion process, thereby giving it the characteristic of being retractile (Fig. 2C).
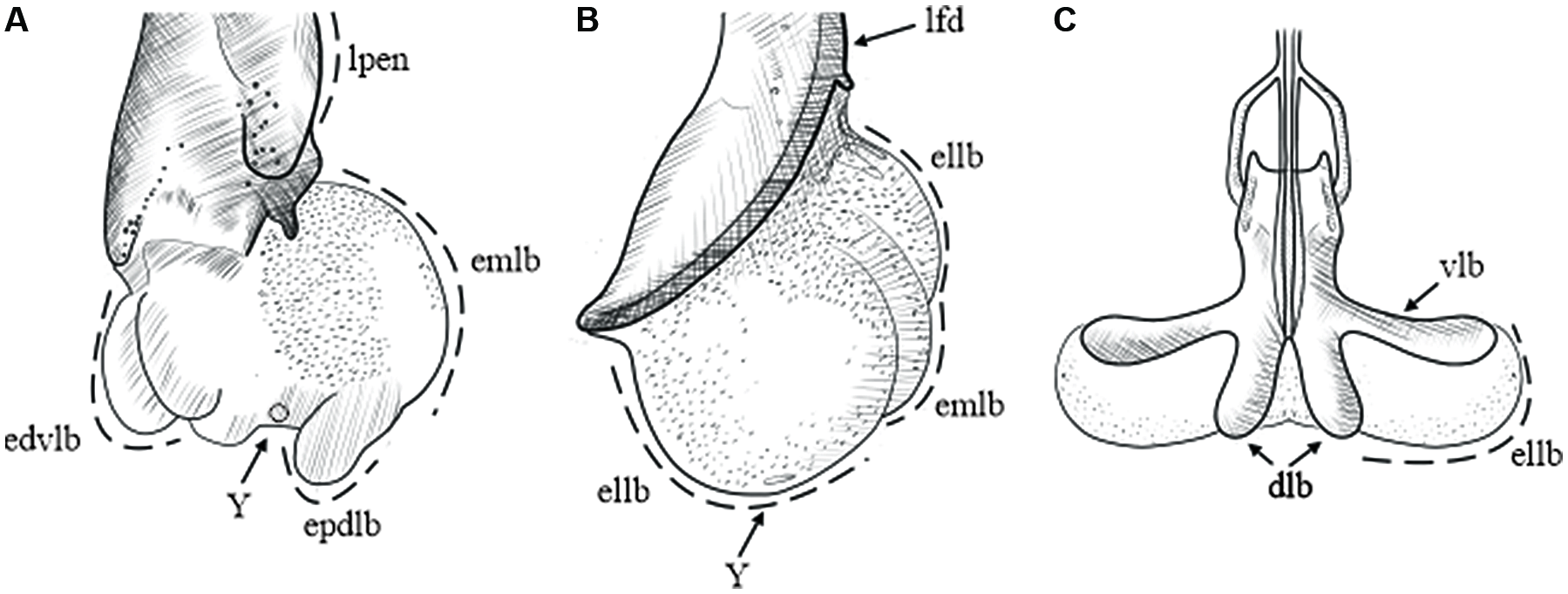
Figure 2. Inflated endophallus patterns in Scolytinae: A, Dendroctonus in lateral view; B, Phloeosinus in lateral view; and C, Ips in dorsal view. dlb, aedeagus’ dorsal lobe; edvlb, endophallus’ distal ventral lobes; ellb, endophallus’ lateral lobes; emlb, endophallus’ median lobe; epdlb, endophallus’ proximal dorsal lobes; lfd, aedeagus’ lateral folds; lpen, lateral lobes of pennis, Y, Y-structure; and vlb, aedeagus’ ventral lobe. (See Supplementary material, File S1 for a list and definitions of the abbreviations.)
We found that the number of endophallus lobes differs among genera and species: Dendroctonus spp. have 3–5 lobes, Phloeosinus spp. have three lobes, and Ips spp. have two (Fig. 2). By comparing the relative position of the lobes, a common morphological pattern in endophalluses became apparent: the presence of a pair of lateral lobes in ventral and lateral views (Fig. 2), whose arrangement and shape differ among genera. The lateral lobes are oval and smaller than the median lobe in Dendroctonus but are rounded and as large or larger than the median lobe in Phloeosinus (Fig 2B); in Ips, only the lateral lobes are present, and their shape is uniform and oval (Fig. 2C). An additional pair of lobes – the proximal dorsal lobes and the distal ventral lobes – can be seen in lateral view in members of the Dendroctonus frontalis complex (Fig. 2A).
Similarities among genera were identified in the present study. In Dendroctonus and Phloeosinus, a median lobe and a yellow pore-like circular structure located in the posterior region of the median lobe are preserved (Figs. 4, 5, 6, 10, 12, 13, 15, and 16), whereas both structures are absent in Ips species (Fig. 2). Cerezke (Reference Cerezke1964, fig. 12) had previously identified the pore-like circular structure as the Y-structure in D. ponderosae. In D. adjunctus, D. mesoamericanus, D. parallelocollis, D. pseudotsugae barragani, and D. valens from Mexico, the Y-structure is poorly sclerotised; thus, it was not possible to obtain clear images that display this element (Fig. 3, 7, 8, 9, and 11). In the present study, spiny sclerite patterns are observed on the outer surface of the lobes in all species. Lateral lobes have evenly arranged conical spines. In Dendroctonus, the median lobe has smaller spines in closely spaced groups, with the spines becoming smaller towards the posterior end of the lobe, and no sclerotisation is observed on the lobe’s ventral side.
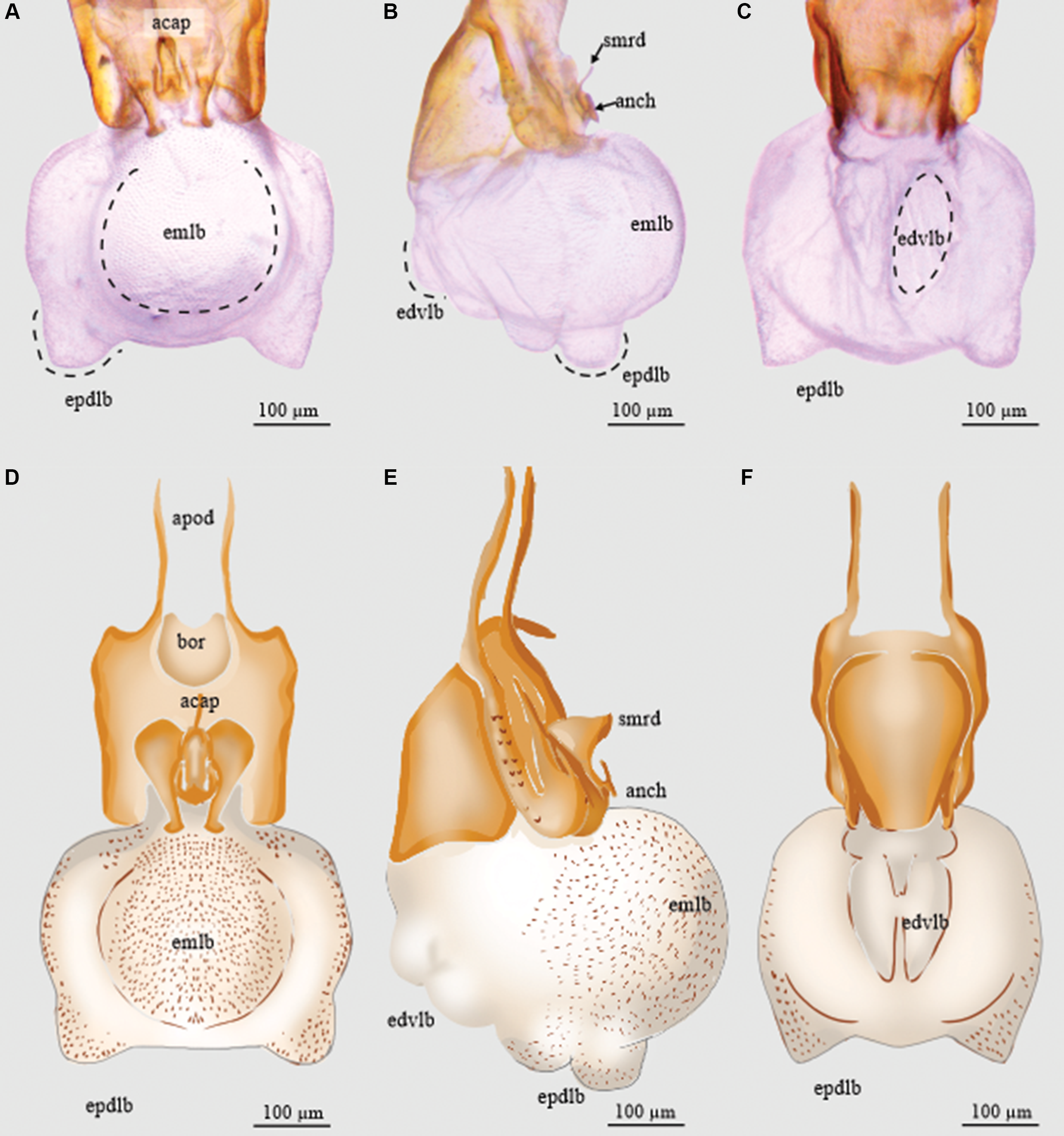
Figure 3. Endophallus of Dendroctonus adjunctus: A and D, dorsal view; B and E, lateral view; and C and F, ventral view. acap, accessory apparatus; anch, anchor; apod, apodemes; bor, basal orifice; edvlb, endophallus’ distal ventral lobes; emlb, endophallus’ median lobe; epdlb, endophallus’ proximal dorsal lobes; and smrd, seminal rod. (See Supplementary material, File S1 for a list and definitions of the abbreviations.)
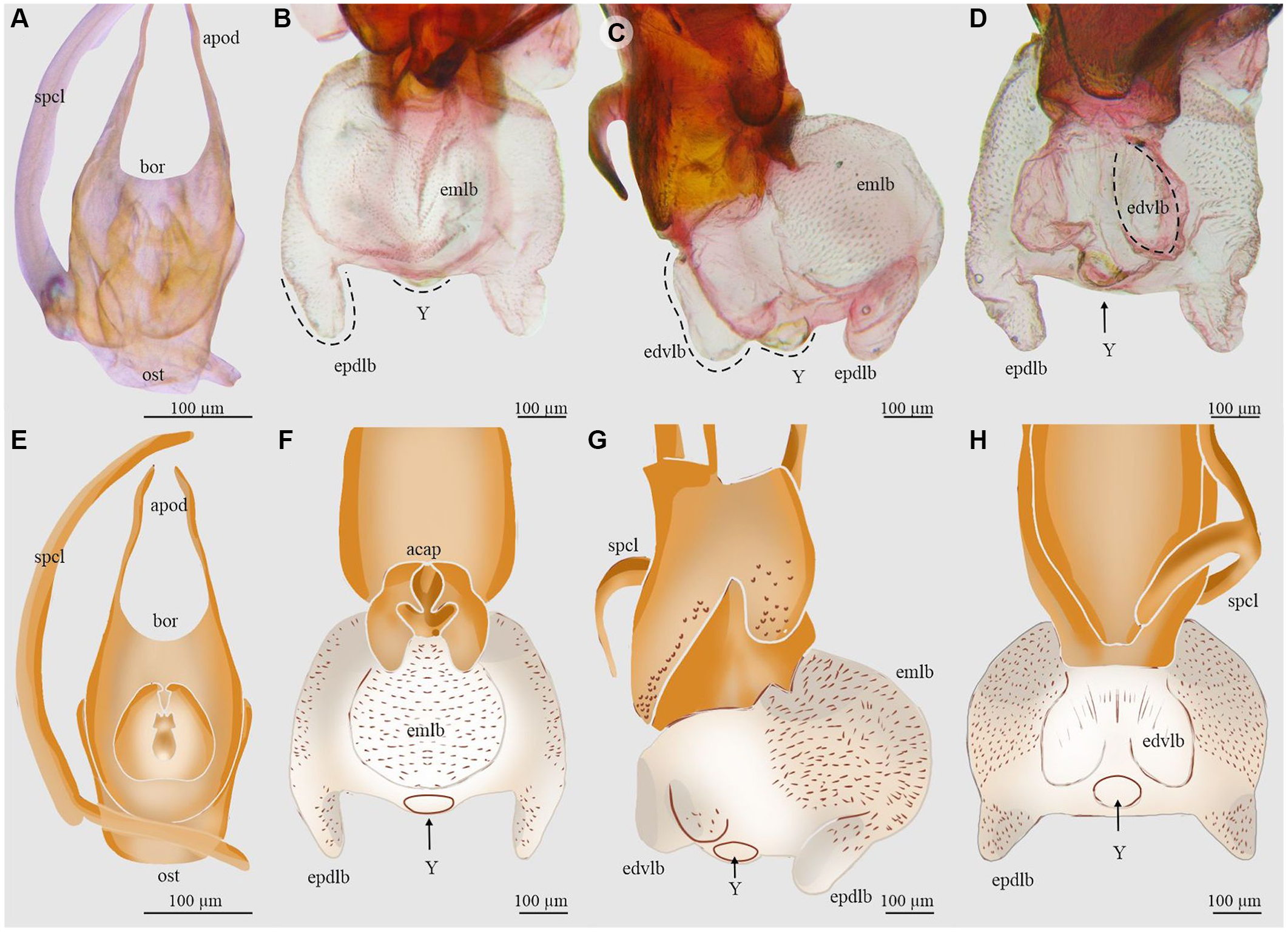
Figure 4. Aedeagus and endophallus of Dendroctonus approximatus: A and E, aedeagus in dorsal view; B and F, endophallus in dorsal view; C and G, endophallus in lateral view; D and H, endophallus in ventral view. acap, accessory apparatus; apod, apodemes; bor, basal orifice; edvlb, endophallus’ distal ventral lobes; emlb, endophallus’ median lobe; epdlb, endophallus proximal dorsal lobes; ost, ostium; spcl, spicule; and Y, Y-structure. (See Supplementary material, File S1 for a list and definitions of the abbreviations.)

Figure 5. Aedeagus and endophallus of Dendroctonus barberi: A and D, aedeagus in dorsal view; B and E, endophallus in dorsal view; C and F, endophallus in ventral view. acap, accessory apparatus; apod, apodemes; bor, basal orifice; edvlb, endophallus’ distal ventral lobes; emlb, endophallus’ median lobe; ost, ostium; epdlb, endophallus’ proximal dorsal lobes; ost, ostium; spcl, spicule; and Y, Y-structure. (See Supplementary material, File S1 for a list and definitions of the abbreviations.)

Figure 6. Aedeagus and endophallus of Dendroctonus frontalis: A and E, aedeagus in dorsal view; B and F, endophallus in dorsal view; C and G, endophallus in lateral view; and D and H, endophallus in ventral view. acap, accessory apparatus; apod, apodemes; bor, basal orifice; ellb, endophallus’ lateral lobes; emlb, endophallus’ median lobe; ost, ostium; smrd, seminal rod; spcl, spicule; and Y, Y-structure. (See Supplementary material, abbreviations S1 for a list and definitions of the abbreviations.)

Figure 7. Aedeagus and endophallus of Dendroctonus mesoamericanus: A and D, aedeagus in dorsal view; B and E, endophallus in dorsal view; and C and F, endophallus in lateral view. acap, accessory apparatus; apod, apodemes; bor, basal orifice; ellb, endophallus’ lateral lobes; emlb, endophallus’ median lobe; ost, ostium; smrd, seminal rod; and spcl, spicule (see Supplementary material, File S1 for a list and definitions of the abbreviations)

Figure 8. Endophallus of Dendroctonus parallelocollis: A and D, aedeagus in dorsal view; B and E, endophallus in lateral view; and C and F, endophallus in ventral view. acap, accessory apparatus; bor, basal orifice; ellb, endophallus’ lateral lobes; emlb, endophallus’ median lobe; smrd, seminal rod; and spcl, spicule. (See Supplementary material, File S1 for a list and definitions of the abbreviations.)
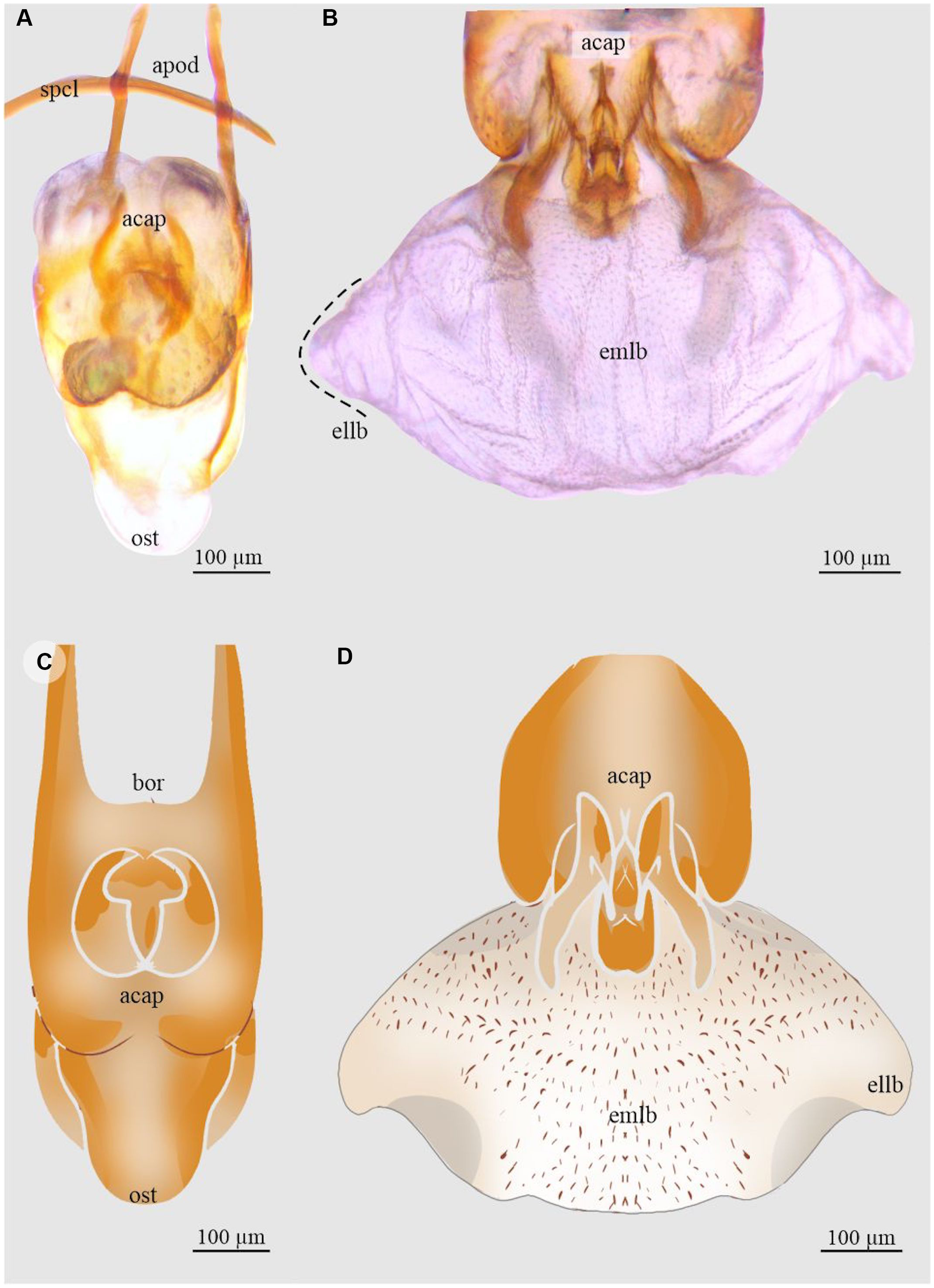
Figure 9. Aedeagus and endophallus of Dendroctonus pseudotsugae barragani: A and C, aedeagus in dorsal view; and B and D, endophallus in dorsal view. acap, accessory apparatus; apod, apodemes; bor, basal orifice; ellb, endophallus’ lateral lobe; emlb, endophallus’ median lobe; ost, ostium; and spcl, spicule. (See Supplementary material, File S1 for a list and definitions of the abbreviations.)

Figure 10. Endophallus of Dendroctonus rhizophagus: A and C, dorsal view; B and D, lateral view displaying the median lobe and the lateral lobes. acap, accessory apparatus; ellb, endophallus’ lateral lobes lobes; emlb, endophallus’ median lobe; and Y, Y-structure. (See Supplementary material, File S1 for a list and definitions of the abbreviations.)

Figure 11. Aedeagus of Dendroctonus valens from Mexico: A and D, dorsal view; B and E, lateral view; and C and F, ventral view. ellb, endophallus’ lateral lobes lobes; and emlb, endophallus’ median lobe. (See Supplementary material, File S1 for a list and definitions of the abbreviations.)

Figure 12. Endophallus of Dendroctonus valens from Honduras: A and D, dorsal view; B and E, lateral view; and C and F, ventral view. acap, accessory apparatus; apod, apodemes; bor, basal orifice; ellb, endophallus’ lateral lobes; emlb, endophallus’ median lobe; ost, ostium; and Y, Y-structure. (See Supplementary material, File S1 for a list and definitions of the abbreviations.)

Figure 13. Aedeagus and endophallus of Dendroctonus vitei: A and D, dorsal view; B and E, lateral view; and C and F, ventral view. acap, accessory apparatus; apod, apodemes; ellb, endophallus’ lateral lobe; emlb, endophallus’ median lobe; and Y, Y-structure. (See Supplementary material, abbreviations S1 for a list and definitions of the abbreviations.)

Figure 14. The structures that conform to the genital organ of Phloeosinus: A and B, aedeagus in dorsal view; C, anchor lateral view; D, seminal rod lateral view; and E, endophallus. acap, ccessory apparatus; anch, anchor, apod, apodemes; bor, basal orifice; enph, endophallus; lfd, aedeagus’ lateral folds; ost, ostium; spcl, spicule; and smrd, seminal rod. (See Supplementary material, File S1 for a list and definitions of the abbreviations.)

Figure 15. Aedeagus and endophallus of Phloeosinus baumanni: A and D, aedeagus in dorsal view; B and E, endophallus in lateral view, C, accessory apparatus in lateral view, and F, Y structure in lateral view. acap, accessory apparatus; ellb, endophallus’ lateral lobes; emlb, endophallus’ median lobe; enph, endophallus; smrd, seminal rod; and Y, Y-structure. (See Supplementary material, File S1 for a list and definitions of the abbreviations.)

Figure 16. Aedeagus and endophallus of Phloeosinus deleoni: A and D, aedeagus in dorsal view; B and E, endophallus dorsal view; and C and F, endophallus in lateral view. acap, accessory apparatus; apod, apodemes; bor, basal orifice; enph, endophallus; ellb, endophallus’ lateral lobes; lfd, aedeagus’ lateral folds; emlb, endophallus median lobe; smrd, seminal rod; spcl, spicule; and Y, Y-structure. (See Supplementary material, File S1 for a list and definitions of the abbreviations.)
Dendroctonus
The endophallus is attached to the anchor arms and below the seminal rod. When pressure is exerted to evert the endophallus, the seminal rod and anchor move forwards, positioning themselves outside the capsule and forming an angle 45 and 90° from its initial position, in lateral view. The size of the endophallus varies among the species, although in D. adjunctus, D. approximatus, and D. pseudotsugae barragani, the internal sac is bigger than the aedeagus, and in D. barberi, D. frontalis, D. mesoamericanus, D. parallelocollis, and D. vitei, the internal is smaller than the aedeagus.
Details for each species are described below.
Dendroctonus adjunctus
The length of the endophallus (321 µm) is more than half the total length of the aedeagus (493 µm). The sac is composed of five lobes: one median lobe, two proximal dorsal lobes (Fig. 3A, D), and two distal ventral lobes (Fig. 3B, E). The median lobe is pronounced, with a rounded shape. The proximal dorsal lobes are small and are located on the apical region of the median lobe (Fig. 3C, F). The distal lobes are smaller than the dorsal proximal ones and are in the ventral region of the median lobe (3B, E). The Y-structure is located in the distal region of the sac (Fig. 3).
Dendroctonus approximatus
The total length of the endophallus (449 µm) almost equals that of the aedeagus (581 µm; Fig. 4A, E). The endophallus is composed of five lobes: a prominent rounded median lobe in dorsal view (Fig. 4B, F), a pair of narrower and shorter distal lobes arising from the ventral surface of the median lobe (Fig. 4C, G), and a pair of proximal lobes arising from the dorsal surface of the median lobe (Fig. 4D, H). The Y-structure is located on the distal region of the sac (Fig. 4B, C, D, F, G, H).
Dendroctonus barberi
The size of the endophallus (490 µm) is similar in proportion to that of the aedeagus (512.54 µm; Fig. 5A, D). It is formed by five lobes: a rounded median lobe in dorsal view (Fig. 5B, E), a pair of wider and larger proximal lobes arising from the ventral surface of the median lobe (Fig. 5C, F), and a pair of narrower, long distal lobes arising from the dorsal surface of the median lobe (Fig. 5B, C, E, F). The proximal and distal lobes are similar in shape, but the proximal lobes are larger than the distal lobes. In the posterior region in the central part of the median lobe, the Y-structure sits on a small evaginated surface of the endophallus (Fig. 5C, F).
Dendroctonus frontalis
The endophallus (140 µm) is shorter than the aedeagus (297.03 µm; Fig. 6A, E). It consists of three lobes: an inconspicuous median lobe and two larger lateral lobes (Fig. 6B, F). In dorsal view, the sizes of the lateral lobes and the median lobe are similar in proportion. The median lobe in the distal region presents the Y-structure (Fig. 6B, C, D, F, G, H). The lateral lobes are covered with small spines evenly distributed over the entire outer surface (Fig. 6C, G).
Dendroctonus mesoamericanus
The length of the endophallus (181 µm) is similar to that of the capsule (172 µm; Fig. 7A, D). It consists of three lobes: a conspicuous median lobe and two well-defined lateral lobes (Fig. 7B, E). The median lobe is bigger than the lateral ones. The Y-structure is in the distal region of the endophallus, between lateral lobes. The surface of the median lobe is covered by large, evenly distributed conical spines, and the lateral lobes are covered by smaller spines (Fig. 7C, F).
Dendroctonus parallelocollis
The length of the endophallus (307 µm) is proportional to that of the capsule (582 µm). It consists of three lobes: a well-defined median lobe in dorsal and lateral views (Fig. 8A, B) and two distal lateral lobes that extend perpendicular to the median lobe and arise from the sides of the posterior region of the median lobe (Fig. 8B, E). The shape of the lateral lobes differs from that of the median lobe because it has some folds that give it an irregular shape that is widest at the base of the lobes (Fig. 8C, F). The Y-structure is in the posterior region of the median lobe. Small conical spines are distributed on the dorsal surface of the median lobe and the anterior region of the lateral lobes, on the median lobe, the density of spines decreases towards the lateral lobes (Fig. 8B, D, E, F).
Dendroctonus pseudotsugae barragani
The length of the endophallus in dorsal view (382 µm) is almost half the total length of the aedeagus (774 µm; Fig. 9A, C). The endophallus of this species consists of three lobes that are poorly differentiated: a prominent median lobe and two small distal lateral lobes (Fig. 9B, D). The surface of the median lobe covers almost the entire endophallus. In the dorsal region, the median lobe is covered by small spines, the density of which decreases towards the lateral lobes (Fig. 9D). The Y-structure is found in the ventral posterior region of the median lobe.
Dendroctonus rhizophagus
A partial inflation of the endophallus was obtained with these specimens. In dorsal view, the endophallus was found to be formed by at least three lobes that resemble a “T” shape (Fig 10A): a poorly developed median lobe and two well-defined distal lateral lobes (Fig. 10A, C). The median lobe is larger than the lateral lobes. The Y-structure sits between the two distal lateral lobes, on the distal region of the endophallus (Fig. 10B, D).
Dendroctonus valens from Mexico
The length of the endophallus is almost proportional to the capsule of the aedeagus (655 µm) and is formed by three lobes that resemble a “Y” shape in ventral view: an inconspicuous median lobe and two prominent distal lateral lobes (Fig. 11A, D). The median lobe is poorly differentiated from the lateral lobes (Fig. 11B, E). Each apex of the lateral lobes ends in a point. The dorsal and lateral surfaces of the endophallus are covered by small spines. The Y-structure sits between the two distal lateral lobes, in the distal region of the endophallus. The density of the spines decreases to the ventral region (Fig. 11D, E, F).
Dendroctonus valens from Honduras
The endophallus is similar to those of D. rhizophagus, formed by three lobes that resemble a short “T” shape in ventral view: a conspicuous median lobe that is divided into two well-defined regions (distal and proximal; Fig. 12A, D); the proximal region is rounded in dorsal view, and the distal region is fused with the two lateral lobes. The apical edge of each lateral lobe is divided into two small lobes that are equal in size and shape, both being rounded (Fig. 12B, E). The median lobe is equal in length to the lateral lobes. The Y-structure sits between the two distal lateral lobes, in the distal region of the endophallus (Fig. 12C, F). The entire surface of the endophallus is covered by small spines, which decrease in density towards the ventral region (Fig. 12D, E, F).
Dendroctonus vitei
The length of the endophallus (208 µm) is more than a half total length of the aedeagus (367 µm). The endophallus is formed by three lobes: a prominent rounded median lobe in dorsal view (Fig. 13A) and two lateral distal lobes that, in dorsal view, are small, very rounded, and are as long as wide (Fig. 13A, D). The lateral lobes are smaller than the median one (Fig. 13B, E). The Y-structure is located in the posterior ventral region of the median lobe (Fig. 13C, F). The entire surface of the endophallus is covered by small spines which decrease in density from the centre of the dorsal region of the median lobe towards the ventral region (Fig. 13D, E, F).
Phloeosinus
The endophallus in this genus is a thin membranous sac, the base of which is attached to the accessory apparatus and the dorsal–distal region of the ostium. The sac surrounds the seminal rod and is fused with the anchor, which is located above the seminal rod (Fig. 14A, B, C, D, E). When the endophallus is everted or inflated, the seminal rod performs a 45° movement. In general, the endophallus of Phloeosinus is formed by three lobes: a median lobe and two lateral lobes that have shapes that are quite uniform in all the species analysed. In the sampled species, the size of the internal sac is similar and not bigger than seminal capsule. In addition, the Y-structure is located in the posterior region of the median lobe and, in lateral view, has a conical shape.
Phloeosinus baumanni
The length of the endophallus in the dorsal view (280 µm) is approximately one-half the total length of the aedeagus (542 µm). The sac consists of a conspicuous median lobe that extends from the basal orifice connected to the accessory apparatus to the ostium (Fig. 15A) and of a pair of poorly marked lateral lobes that protrude further than the median lobe and expand beyond the ostium (apical region of the aedeagus). The shape of the endophallus lobes is rounded (Fig. 15B, E). The lateral lobes are not well differentiated from the median lobe, and they appear to be the same size as the median lobe in lateral view (Fig. 15A, D). All lobes are covered by small spines which are denser at the base (anterior region) near the accessory apparatus (Fig. 15C). The Y-structure is located in the posterior region of the median lobe (Fig. 15D, E, F).
Phloeosinus deleoni
The length of the endophallus (328.58 µm) is more than half of the total length of the aedeagus (537.35 µm) in dorsal view (Fig.16A). The median lobe is prominent compared to the lateral lobes (Fig. 16B, E). In the posterior region, the median lobe is rounded, and the lateral lobes display oval edges (Fig. 16C, F). The Y-structure is located in the median lobe apex (Fig. 16B, C, E, F). All lobes have spines in their dorsal regions; the spines are denser at the lobes’ bases (next to the accessory apparatus) and decrease in density towards the anterior regions (Fig. 16D, E).
Phloeosinus tacubayae
The length of the endophallus (294.33 µm) is approximately three-quarters of the total length of the aedeagus (441.09 µm) in dorsal view (Fig. 17A). The endophallus consists of three inconspicuous lobes (Fig. 17B, D). The median lobe is prominent compared to the lateral lobes and is rounded in the anterior region (Fig. 17C, E). The two lateral lobes are poorly differentiated from the median lobe and are much less evaginated than the median lobe – in some views, the endophallus appears to be unilobate (Fig. 17B–E). In the apex of the median lobe is a protruding Y-structure (Fig. 17C, D, E). Spines cover the anterior region near the base of the endophallus, which become less dense towards the posterior–apical region (Fig. 17B–E).

Figure 17. Aedeagus and endophallus of Phloeosinus tacubayae: A, aedeagus dorsal view; B and D, endophallus dorsal view; and C and E, endophallus lateral view. acap, accessory apparatus; apod, apodemes; bor, basal orifice; ellb, endophallus’ lateral lobes; emlb, endophallus’ median lobe; enph, endophallus; lfd, aedeagus’ lateral folds; smrd, seminal rod; spcl, spicule; and Y, Y-structure. (See Supplementary material, File S1 for a list and definitions of the abbreviations.)
Phloeosinus serratus
For this species, specimens from three populations collected from different Cupressaceae host plants, Hesperocyparis arizonica (Miller) Bartel, Juniperus coahuilensis (Martínez) Gaussen ex. R.P. Adams, and Juniperus saltillensis M.T. Hall, were studied. Specimens from the three hosts present conspicuous morphological differences in external and female genital morphology; however, of the three populations of P. serratus, the characteristics of the endophallus were similar (Fig. 18): the endophallus consists of a median lobe and two smaller lateral lobes. In the region near the accessory apparatus (Fig. 18B–G), the surface of the endophallus is covered by small spines, which decrease in density towards the sides (Fig 18G). The Y-structure is in the apical and central region of the endophallus and protrudes from the median lobe (Fig. 18H, I).

Figure 18. Aedeagus and endophallus of Phloeosinus serratus: A, C, and E, aedeagus dorsal view; B, D, F, and G, endophallus lateral view; H and I, Y-structure in dorsal and lateral view, respectively. Specimens from Hesperocyparis arizonica (A, B); Juniperus coahuilensis (C, D); and J. saltillensis (E, F, and G). acap, accessory apparatus; apod, apodemes; bor, basal orifice; ellb, endophallus’ lateral lobes; emlb, endophallus’ median lobe; enph, endophallus; lfd, aedeagus’ lateral folds; ost, ostium; smrd, seminal rod; spcl, spicule; and Y, Y-structure. (See Supplementary material, File S1 for a list and definitions of the abbreviations.)
Ips
The endophallus is a retractile eversible sac and has two lateral lobes. The sac is attached to the dorsal lobes of the aedeagus in the median part of the dorsal region (junction point of both lobes) and is joined on the sides with the ventral lobes (Figs. 19, 20, and 21). The seminal trough is located in the central part of the aedeagus and, at the time of inflation, descends slightly towards the posterior part of the endophallus (Fig. 19A). When inflation is performed, the ventral lobes expand sideways, allowing the eversion of the endophallus: the movement resembles the opening of a fan (Figs. 20B and 21D, E). However, when the pressure exerted during inflation is removed, the endophallus retracts, and the ventral lobes return to their initial positions. In the two species analysed, the size of the lobes is proportional to the length of the ventral lobes of the aedeagus.

Figure 19. Complete genital organ in dorsal view of Ips: A, aedeagus; B, spicula; C, tegmen; D, seminal trough, E, retracted endophallus in dorsal view, and F, retracted endophallus in ventral view. apod, apodemes; dlb, aedeagus’ dorsal lobe; enph, endophallus; smt, seminal trough; and vlb, aedeagus’ ventral lobe. (See Supplementary material, File S1 for a list and definitions of the abbreviations.)
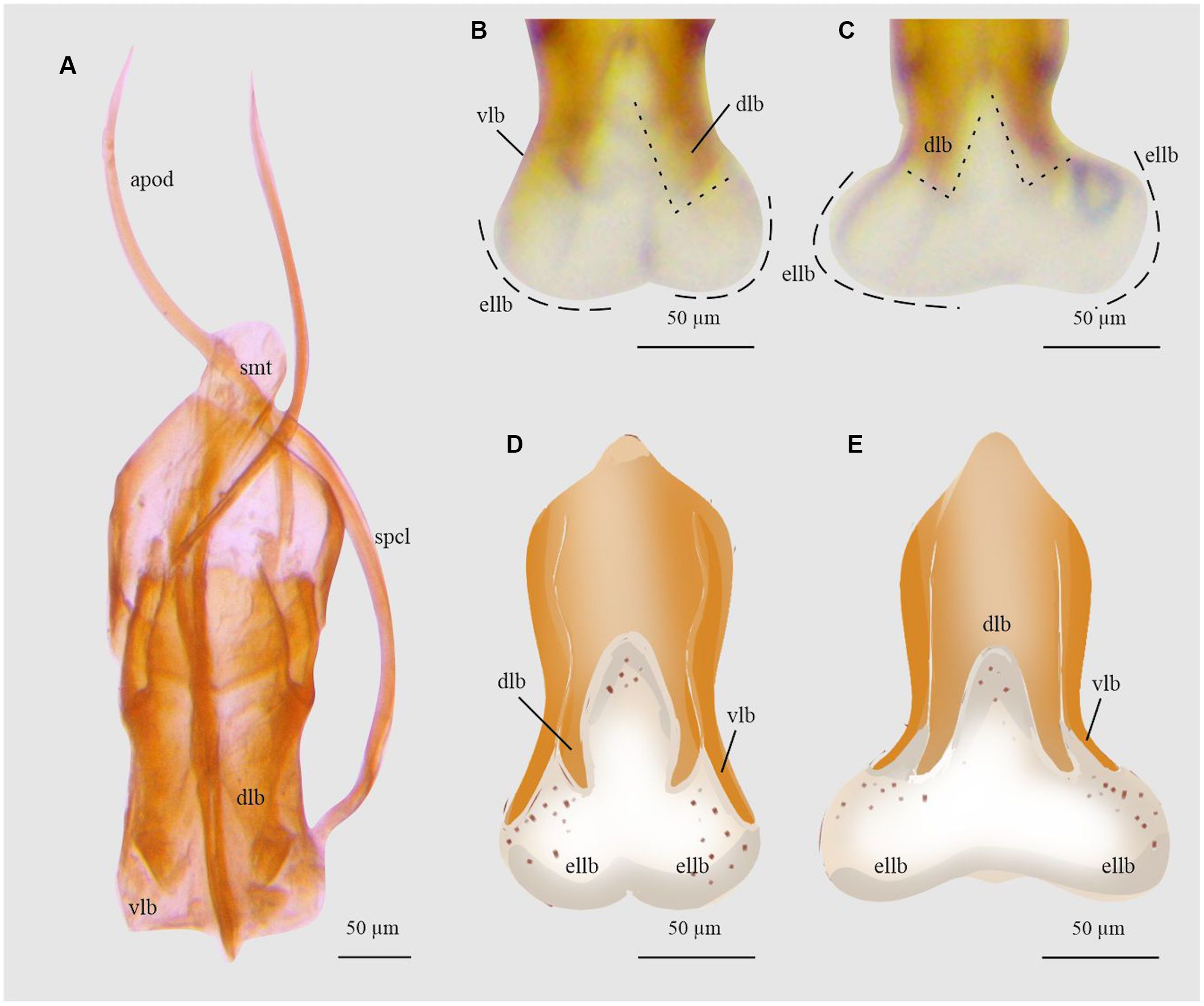
Figure 20. Aedeagus and endophallus of Ips calligraphus: A, aedeagus dorsal view; B and D, endophallus dorsal view; and C and E, endophallus ventral view. apod, apodemes; dlb, aedeagus’ dorsal lobe; ellb, endophallus’ lateral lobes; smt, seminal trough; spcl, spicule; and vlb, aedeagus’ ventral lobe. (See Supplementary material, File S1 for a list and definitions of the abbreviations.)

Figure 21. Aedeagus and endophallus of Ips lecontei in dorsal view: A, aedeagus; B, endophallus. apod, apodemes; dlb, aedeagus’ dorsal lobe; ellb, endophallus’ lateral lobes; smt, seminal trough; and vlb, aedeagus’ ventral lobe. (See Supplementary material, File S1 for a list and definitions of the abbreviations.)
Ips calligraphus
In this species, when the endophallus is inflated and everted, the ventral and dorsal lobes of aedeagus are slightly moved together with the endophallus lobes (Fig. 20D, E). The point of the ventral lobe expands poorly to the sides, forming an angle of less than 45° to the dorsal lobes, and the dorsal lobes are slightly separated (Fig. 20B, C). The endophallus lateral lobes are as long as wide as and longer than the aedeagus lobes (Fig. 20D, E).
Ips lecontei
When the endophallus is inflated and everted in this species, the ventral and dorsal lobes of aedeagus are strongly moved together with the endophallus lobes (Fig. 21B). The ventral lobe expands sideways, forming a 90° angle to the dorsal lobes, and the dorsal lobes separate slightly between them (Fig. 21B). The endophallus lateral lobes are longer than wide, their length is slightly higher than the ventral lobe, and their width is equivalent to dorsal lobe length (Fig. 21B).
Discussion
In this study, Van Dam’s (Reference Van Dam2014) technique was modified for the study of the endophallus of Scolytinae, allowing the description of the morphology of the internal sac and the inflation patterns in 16 species from Dendroctonus, Ips, and Phloeosinus. The species differ in endophallus attachment types and display two distinct inflation and retraction mechanisms. Our results show that the internal sac is a potentially useful tool for Scolytinae taxonomy, as each genus appears to have its own morphological patterns, particularly in Dendroctonus, where differences and similarities are more pronounced in closely related species.
Previous studies that use the endophallus generated several techniques to facilitate the organ’s processing and eversion and to allow its conservation once inflated (Berlov Reference Berlov1992; Uliana and Sabatinelli Reference Uliana and Sabatinelli2010; Janovska et al. Reference Janovska, Anichtchenko and Erwin2013; Daccordi et al. Reference Daccordi, Bollino and Vela2020). An important issue with those methods, including the most widely used technique, the Berti–Vachon method (Bontems Reference Bontems2013), is their applicability in small and pin-mounted specimens. Van Dam (Reference Van Dam2014) presented an easy technique for endophallus eversion that is fast and can be used in various taxa within Curculionidae. The modifications to this technique that were tested in the present study, including the rehydration of dry specimens, the two digestion steps (enzyme and KOH), the dilution of inflation fluid, and the modifications of the tools (forceps and syringes), together enabled satisfactory results with Scolytinae specimens that were preserved in different ways. First, we were able to inflate the endophalluses of large species, such as D. rhizophagus (5.0–6.3 mm) and D. valens (5.3–8.3 mm), and small ones, such as D. frontalis (2.0–3.2 mm) and P. tacubayae (1.9–2.4 mm), which represent a range in body size among the family. Also, the technique worked properly in both fresh and pin-mounted specimens and permitted relatively fast endophallus inflation, facilitating the analysis of at least two specimens per species.
Two techniques have been implemented in other curculionids for endophallus eversion. The first is the Berti–Vachon (Bollino and Sandel Reference Bollino and Sandel2017; Meregalli et al. Reference Meregalli, Boriani, Taddei, Hsu, Tseng and Mouttet2020), and the second and more recent is the Van Dam (Reference Van Dam2014) technique. Both consist of three general steps: (1) the dissection and enzymatic digestion of the aedeagus, (2) the inflation of the endophallus, and (3) the preparation of the endophallus for photography. In Scolytinae specimens, these three steps permit relatively good results in fresh specimens; however, in specimens that have been preserved in alcohol for several years and in those that are dry-mounted, muscle tissues are hard, and dissecting without damaging the abdominal segments and aedeagus pieces is difficult. As our results show, including a prior rehydration step that uses a “brine” softening solution softens the cuticular membranes and muscle fibres and allows cleaner dissections and isolation of the aedeagus with greater ease and integrity.
In other curculionids, the pancreatin enzymatic solution by itself digests tissues within a couple of hours (Van Dam Reference Van Dam2014). However, our results indicate that, in dry-preserved specimens or those with highly chitinised structures – such as D. adjunctus, D. approximatus, D. parallelocollis, D. rhizophagus, D. valens, and P. baumanni – the pancreatin solution alone is insufficient for soft tissue degradation and that adding a second digestion step consisting of a low concentration solution of KOH is required. The use of K-Y gel, which is transparent, as a filling substance allows the details of the endophallus to be observed, unlike the use of opaque substances such as toothpaste (Uliana and Sabatinelli Reference Uliana and Sabatinelli2010; Janovska et al. Reference Janovska, Anichtchenko and Erwin2013), which mask the endophallus’s sclerotised structures. However, we found it necessary to dilute the gel (50%) because the viscosity provided by the manufacturer prevented the gel from flowing through and inflating the structures. Despite the decreased viscosity, the gel strengthened the sampled tissues and allowed their manipulation with a low risk of collapse, as can happen when other fluids such as alcohol and glycerin are used (Yamasako and Ohbayashi Reference Yamasako and Ohbayashi2011).
One of the key points to achieving successful endophallus inflation is a good coupling and clamping of the base of the aedeagus with the forceps. Van Dam (Reference Van Dam2014) suggests using microvascular corneal forceps (no. 18155-13); however, the size of the aedeagus in Scolytinae is small, and these forceps are coarse and stiff, which hinders the manipulation of the aedeagus and therefore the inflation process. In the case of very small specimens such as Scolytinae, we recommend using very fine-tipped forceps because this allows better control when manipulating the sample during the inflation process. Also, we recommend using 70% alcohol to preserve the inflated samples: even though the duration of preservation is not yet defined, the method seems to be efficient.
Anatomy and nomenclature
Prior studies on the anatomy of the endophallus in the Coleoptera have focused mainly on descriptions of the organ’s lobes and sclerotised structures, especially in related or individual species (Bollino and Ruzzante Reference Bollino and Ruzzante2015; Bollino and Sandel Reference Bollino and Sandel2017; Meregalli et al. Reference Meregalli, Boriani, Taddei, Hsu, Tseng and Mouttet2020). Génier (Reference Génier2019) proposes standardising the nomenclature of several sclerosed elements of the insect endophallus; however, the terms used to name and describe the aedeagal lobes remain unhomogenised mainly because the lobes’ numbers, positions, and shapes display great intra- and interspecific variation among taxa (Bollino and Ruzzante Reference Bollino and Ruzzante2015; Bollino and Sandel Reference Bollino and Sandel2017; Meregalli et al. Reference Meregalli, Boriani, Taddei, Hsu, Tseng and Mouttet2020). The assignment of names to and descriptions of lobes has been based mainly on their position vis-a-vis the aedeagus – for example, the basal lobe, the median lobe, the lateral lobes, the apical lobe, and so on (Yamasako and Ohbayashi Reference Yamasako and Ohbayashi2011; Daccordi et al. Reference Daccordi, Bollino and Vela2020; Meregalli et al. Reference Meregalli, Boriani, Taddei, Hsu, Tseng and Mouttet2020). In the present study, the descriptions of the lobes and structures in their relation to the endophallus were made according to the nomenclature described by Cerezke (Reference Cerezke1964), whose criteria for naming the lobes were also based on their relational positions. The endophallus elements previously described for Dendroctonus ponderosae were the median lobe, the lateral lobes, the Y-structure, and the spines. In the descriptions made as part of the present study, the terms were adopted for the three genera that were analysed, and a general pattern of arrangement of two lateral lobes was recognised in Dendroctonus, Ips, and Phloeosinus. Although the scope of the nomenclature proposed in the present study could not be extrapolated to the entire subfamily, by the representation of a few taxa (Bollino and Sandel Reference Bollino and Sandel2017; Meregalli et al. Reference Meregalli, Boriani, Taddei, Hsu, Tseng and Mouttet2020), our data from three genera from different tribes (Hylurgini, Phloeosinini, and Ipini, sensu Hulcr et al. Reference Hulcr, Atkinson, Cognato, Jordal, McKenna, Vega and Hofstetter2015) suggest that within Scolytinae, at least two lobes are maintained, and their numbers, positions, and shapes vary among genera and tribes. Therefore, some terms such as “median lobe” and “lateral lobes” apply to the whole group.
Another important point is the circular structure, or Y-structure, previously defined by Cerezke (Reference Cerezke1964) in the genus Dendroctonus and now described for the genus Phloeosinus. Although this structure is not the same in both genera, it is similar in shape and location and is probably a maintained feature within the subfamily Scolitynae.
Taxonomy
The endophallus is a key structure in the taxonomy and evolution of Coleoptera (Zhou et al. Reference Zhou, Zhou, Ski and Beutel2020; Bieńkowski Reference Bieńkowski2021) and has been widely incorporated into species descriptions, phylogenetic reconstructions, and evolutionary biology studies because attributes present on the endophallus surface, such as sclerites, provide a rich source of taxonomic characteristics (Zunino and Halffter Reference Zunino and Halffter1988; Coca-Abia Reference Coca-Abia2007; Medina et al. Reference Medina, Molano and Scholtz2013; Roggero et al. Reference Roggero, Barbero and Palestrini2015; Uliana and Sabatinelli Reference Uliana and Sabatinelli2010).
The results of the present study support the use of endophallus morphology as a tool in taxonomy for the Scolytinae because each genus displays a particular pattern in the shape of the endophallus and because the arrangement, number, size, and shape of its lobes differ among genera and species. The inflation patterns display two different types of attachment between the aedeagus and the internal sac: in Phloeosinus, the base is attached to ventral folds around the ostium, and in Dendroctonus and Ips, the attachment of the sac membrane is by three or four distal lobes, respectively. In addition, the genera also differed in how the sacs retracted: in Ips specimens, the sac is retracted by the resistance applied by the ventral and dorsal lobes of the aedeagus, and in Dendroctonus and Phloeosinus, no mechanism of retraction is apparent, suggesting that retraction is due solely to the lack of seminal fluid pressure and muscular action (Cerezke Reference Cerezke1964).
In Dendroctonus, the taxa with the most specimens examined in this study, the morphological patterns of the endophallus also suggest their value as phylogenetic characteristics. Endophallus similarities among species extend to those of the groups or complexes, as is supported with morphological, karyological, biological, and molecular characteristics as noted in Armendáriz-Toledano et al. (Reference Armendáriz-Toledano, Niño, Macías Sámano and Zúñiga2014, Reference Armendáriz-Toledano, Niño, Sullivan, Kirkendall and Zúñiga2015), Víctor and Zúñiga (2015), Godefroid et al. (Reference Godefroid, Meseguer, Sauné, Genson, Streito and Rossi2019), García-Román et al. (Reference García-Román, Armendáriz-Toledano, Valerio-Mendoza and Zúñiga2019, Reference García-Román, Ramírez-Reyes and Armendáriz-Toledano2022), Sullivan et al. (Reference Sullivan, Grady, Hofstetter, Pureswaran, Brownie, Cluck and Zúñiga2021), and Ramírez-Reyes et al. (Reference Ramírez-Reyes, Armendáriz-Toledano and Rodríguez2023).
The species D. vitei, D. mesoamericanus, D. frontalis, D. approximatus, and D. adjunctus are grouped within the Dendroctonus frontalis complex (Lanier Reference Lanier1987; Víctor and Zúñiga Reference Víctor and Zúñiga2016; Ramírez-Reyes et al. Reference Ramírez-Reyes, Armendáriz-Toledano and Rodríguez2023). In the present study, this group was characterised by a rounded median lobe and two small lateral lobes arising in the dorsal–apical region of the median lobe. In D. frontalis, D. mesoamericanus, and D. vitei, members of Dendroctonus frontalis complex sensu stricto (Lanier Reference Lanier1987) or clades IV and V (Ramírez-Reyes et al. Reference Ramírez-Reyes, Armendáriz-Toledano and Rodríguez2023), three similar lobes in position are present, but in D. adjunctus, D. approximatus and D. barberi, the other members of this complex corresponding to clade III (Ramírez-Reyes et al. Reference Ramírez-Reyes, Armendáriz-Toledano and Rodríguez2023) share five lobes that are similar in position. However, in comparing the morphological patterns within these clades, discordances are observed in closely related species; for example, the endophallus of D. vitei is more similar to that of D. mesoamericanus than to D. frontalis, even though the latter two are sister species.
Members of the D. valens group also share endophallus similarities: D. rhizophagus and D. valens display three lobes, resembling a “T” and “Y” shape in dorsal view, a poorly developed median lobe, and two well-defined distal lateral lobes. Differences in lobe shape were observed among specimens of D. valens from Honduras and Mexico: the endophallus of D. valens from Honduras is more similar to those in D. rhizophagus: in both species, it is formed by three lobes resembling a “T” shape in ventral view, with the apical lateral lobe divided into two rounded small lobes equal in size and shape, whereas D. valens from Central Mexico displays an endophallus formed by three lobes resembling a “Y” shape in ventral view and two prominent distal lateral lobes with the apices ending in a point.
These morphological differences are consistent with the hypothesis that the Central American populations of D. valens correspond to a different species (Cai et al. Reference Cai, Cheng, Xu, Duan and Kirkendall2008; Armendáriz-Toledano and Zúñiga Reference Armendariz-Toledano and Zúñiga2017), which was previously recognised as D. beckeri by Thatcher (Reference Thatcher1954) and later synonymised with D. valens (Wood Reference Wood1963).
Dendroctonus pseudotsugae barragani and D. parallelocollis present well-defined morphological patterns, distinct from those of D. frontalis and D. valens groups. The second species displays some shared attributes with the D frontalis complex: two small lateral rounded lobes attached to the distal end of the median lobe. In the most recently phylogeny, D. parallelocollis is the sister species of the D. frontalis complex (Ramírez-Reyes et al. Reference Ramírez-Reyes, Armendáriz-Toledano and Rodríguez2023).
Sasabe et al. (Reference Sasabe, Takami and Sota2010) have proposed a possible lock-and-key role between the endophallus and bursa copulatrix in related species with variable endophallus morphology; however, Janovska et al. (Reference Janovska, Anichtchenko and Erwin2013) questions this theory for genera in which the endophallus is highly uniform. In the present study, we observed that the shape of the endophallus varies within the genus Dendroctonus, indicating that the lock-and-key condition may apply; however, examination of the shape and attributes that make up the bursa copulatrix is needed to corroborate whether the two structures complement each other.
In the genus Phloeosinus, the morphological differences among species are less evident and mostly are found in the shape of the endophallus lobes; however, more sister species pairs need to be studied to corroborate the lobes’ taxonomic potential. Finally, the two Ips spp. examined in the present study display a less complex endophallus morphological pattern, with only two lateral lobes, which differ in the two species. Further analysis is required in other species of Ips to determine whether a pattern exists and to identify another, denser substance to inflate the endophallus that prevents its retraction and maintains its shape for a longer time to facilitate its anatomical study.
Although the present study elucidates the attributes of the endophallus and the importance of its study within the subfamily Scolytinae, further investigation into the possible evolutionary patterns of this structure is necessary to reveal how it may further the development of phylogenetic inferences.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.4039/tce.2024.27.
Acknowledgements
We thank the following institutions for funding this research: PAPIIT–UNAM, (IA203122, IN223924), and Consejo Nacional de Humanidades Ciencias y Tecnologia CONAHCYT Fronteras de la Ciencia (139030). Alice Nelly Fernández-Campos and Ana Valería Guzmán-Robles are students at Programa de Posgrado en Ciencias Biológicas, Universidad Nacional Autónoma de México and received fellowships 1146407 and 631009, respectively, from CONAHCYT. Francisco Armendáriz-Toledano and Gerardo Cuéllar-Rodríguez are members of Sistema Nacional de Investigadores-CONAHCYT.

























