Introduction
Invasive alien species have been a major ecological concern for the last few decades, and most introductions of such species are anthropogenic (Lowry et al. Reference Lowry, Rollinson, Layboum, Scott, Aiello-Lammens and Gray2013). They can have an important impact on local biodiversity and also on ecosystem services and human well-being (McGeoch et al. Reference McGeoch, Butchart, Spear, Marais, Kleynhans and Symes2010; Shackleton et al. Reference Shackleton, Shackleton and Kull2018). Introduced species are often more disruptive in novel territories than in their native ranges through different mechanisms, including lower plant defense (Desurmont et al. Reference Desurmont, Donoghue, Clement and Agrawal2011) and lower susceptibility to natural enemies (enemy-release hypothesis; reviewed by Colautti et al. Reference Colautti, Ricciardi, Grigorovich and MacIsaac2004 and Heger and Jeschke Reference Heger and Jeschke2014), that remove checks to population growth and damage. The emerald ash borer, Agrilus planipennis Fairmaire (Coleoptera: Buprestidae), is a recent example of an invasive insect having a higher impact in its introduced range both in Europe and in North America compared to in its native range in Asia (Poland and McCullough Reference Poland and McCullough2006; Orlova-Bienkowskaja Reference Orlova-Bienkowskaja2013). Two other examples of forest exotic pest species having higher impacts in their introduced ranges in North America are the pine shoot beetle, Tomicus piniperda (Linnaeus) (Coleoptera: Scolytidae) (reviewed in Poland and Haack Reference Poland and Haack2003), and the brown longhorn spruce beetle, Tetropium fuscum (Fabricius) (Coleoptera: Cerambycidae) (Smith and Hurley Reference Smith and Hurley2000).
In North America, elm trees are threatened by Dutch elm disease, which was accidentally introduced to Canada in 1945 (reviewed by Hubbes Reference Hubbes1999). This disease, caused by the fungus Ophiostoma ulmi (Buisman) Nannfeldt (Ophiostomataceae) and carried by two bark beetles, Hylurgopinus rufipes (Eichhoff) and Scolytus multistriatus (Marsham) (Coleoptera: Curculionidae), affects all three indigenous elm species in Canada – Ulmus americana Linnaeus, U. rubra Muhlenberg, and U. thomasii Sargent (Ulmaceae) – as well as exotic elm species. The disease causes rapid decline of elms and their eventual death. In the city of Toronto, Ontario, Canada, the elm population was reduced by 80% because of the disease (Huntley Reference Huntley1982, cited by Hubbes Reference Hubbes1999). However, some municipalities, including Québec City, Québec, Canada (which has American elm (U. americana) – also called white elm – as its emblem tree), have management programmes to rapidly eradicate new infestations, thus allowing elm to remain prominent in the urban landscape.
In addition to Dutch elm disease, Canadian elms are affected by several native insect pests and also alien insect species, including the recently introduced elm leafminers Orchestes steppensis Korotyaev (Coleoptera: Curculionidae), Stigmella multispicata Rocienė & Stonis (Lepidoptera: Nepticulidae), and Fenusa pusilla (Serville) (= F. ulmi Sundevall) (Hymenoptera: Tenthredinidae) (Sweeney et al. Reference Sweeney, Anderson, Webster and Neville2012; Miller and Ware Reference Miller and Ware2014; van Nieukerken et al. Reference van Nieukerken, Gilrein and Eiseman2018). The arrival of another invasive species attacking elms could thus have an important impact in North America. The elm zigzag sawfly, Aproceros leucopoda Takeuchi (Hymenoptera: Argidae), is indigenous to east Asia. It was reported for the first time in Central Europe in 2003 (Blank et al. Reference Blank, Hara, Mikulás, Csóka, Ciornei and Constantineanu2010), although an earlier, undetected presence seems plausible. The sawfly spread rapidly across a vast geographical area of western Eurasia and now ranges from England (Royal Botanic Garden Edinburgh 2018) in the west to central Russia (Sundukov Reference Sundukov2017) and Kazakhstan (Ashikbayev et al. Reference Ashikbayev, Mukhamadiyev, Mengdibayeva, Temirzhanov and Kuanyshbaev2018) in the east.
The life history of A. leucopoda favours successful establishment in new areas, whence it can spread rapidly (Blank et al. Reference Blank, Hara, Mikulás, Csóka, Ciornei and Constantineanu2010): it potentially can have four to six generations per year, leading to rapid population growth; it reproduces parthenogenetically without males – that is, it reproduces by thelytoky (all eggs are unfertilised and produce females) and no male has ever been observed; and it actively can disperse 45–90 km per year (Blank et al. Reference Blank, Hara, Mikulás, Csóka, Ciornei and Constantineanu2010, Reference Blank, Köhler, Pfannenstill, Neuenfeldt, Zimmer and Jansen2014). Its relatively low supercooling point, which varies between –12 °C and –24 °C in Europe (Vétek et al. Reference Vétek, Fekete, Ladányi, Cargnus, Zandigiacomo and Oláh2020), also suggests that it is able to survive Canadian winters.
Blank et al. (Reference Blank, Hara, Mikulás, Csóka, Ciornei and Constantineanu2010) examined the bionomics of this species. Elm zigzag sawfly females lay between 7 and 49 eggs individually on the edges of elm leaves. The young larvae hatch after four to eight days and start feeding from the edge of the leaf in a zigzag pattern between two veins towards the midrib. Later-instar larvae can eventually consume the entire leaf, leaving only the midrib. After 15–18 days, the last (sixth) instar larvae form loose cocoons, easily recognised, underneath the leaves. Adults emerge quickly (two to three days) after cocoon formation. Females can start laying eggs immediately without mating, which contributes to rapid population increase. Summer and winter cocoons are dimorphic: the former are as described above, whereas the latter have more solid walls that better protect larvae during winter on the ground. Such “winter” cocoons have been reported to be produced throughout the summer, which may indicate an adaptation that ensures some individuals will be ready for winter even if cold temperatures arrive unexpectedly. Adults from summer cocoons tend to be paler and have shorter genae than adults from winter cocoons.
Aproceros leucopoda attracted attention as a pest of elms in China and Japan, where the species is native (Wu Reference Wu2006; Blank et al. Reference Blank, Hara, Mikulás, Csóka, Ciornei and Constantineanu2010; Cao et al. Reference Cao, Lin, Wu, Zhang and Zhao2011). In Europe, serious damage has been reported, in particular in arid regions of Russia in the northwest Caucasus Range, where the introduced Siberian elm (U. pumila Linnaeus) has been planted in wind shelters along streets and arable areas (e.g., Martynov and Nikulina Reference Martynov and Nikulina2017; Belitskaya and Gribust Reference Belitskaya and Gribust2019). The elm zigzag sawfly can completely defoliate a tree (Zandigiacomo et al. Reference Zandigiacomo, Cargnus and Villani2011; Blank et al. Reference Blank, Köhler, Pfannenstill, Neuenfeldt, Zimmer and Jansen2014), and although attacked trees can produce a second batch of leaves and thus survive, their growth can be affected. No mortality has been reported at the time of writing (Zandigiacomo et al. Reference Zandigiacomo, Cargnus and Villani2011). However, the sawfly’s presence, like other pests, could exacerbate the impact of Dutch elm disease on trees affected by both (Büchel et al. Reference Büchel, Fenning, Gershenzon, Hilker and Meiners2015). Its presence in North America could thus have important impacts, especially for municipalities with programmes protecting against Dutch elm disease.
In summer 2020, the first observation of A. leucopoda in North America was reported on the community science website, iNaturalist (Hogue Reference Hogue2020). The objectives of the present study were: (1) to confirm species identification following onsite specimen collection; (2) to characterise the site of the first observation; (3) to solicit additional observations through social media and community science; and (4) to make a first assessment via molecular analysis of the likely geographic origin of this introduction.
Material and methods
Initial observation and field mission
The first signs of defoliation were observed on 30 July 2020 and published the next day on the community science website, iNaturalist (Hogue Reference Hogue2020). The photo published alongside the report was originally added to the “Leafminers of North America” project on iNaturalist, where it was recognised as depicting not a leaf mine but potential evidence of A. leucopoda. This observation was brought to the attention of entomologists in the province of Québec via email by the observer. The site was visited on 27 August 2020 for thorough investigation and specimen collection. Collected specimens were sent to the Canadian Food Inspection Agency Entomology Lab in Ottawa, Ontario, Canada for identification using the key from Blank et al. (Reference Blank, Hara, Mikulás, Csóka, Ciornei and Constantineanu2010). Three larvae and a single adult (emerged from a cocoon during transportation back to the lab) were deposited as voucher specimens in the entomological collection of the Canadian Food Inspection Agency Entomology Lab in Ottawa (CFIA#20-00981).
Characterisation of the first reported site
Site characteristics
The following protocol was adapted from Canada’s National Forest Inventory (Natural Resources Canada 2008). Six 0.01-ha plots (5.64-m radius) were established in Sainte-Martine, Québec, where the first observation was reported alongside a road, to characterise the site composition. These plots were set up in three pairs, each pair having one plot on each side of the road (Fig. 1). Within these plots, tree species, diameter at breast height, and crown class (dominant, codominant, intermediate, and suppressed) were noted for each tree that was larger than 9 cm in diameter at breast height. Starting from the centre of each plot, the same measurements were made on trees that were smaller than 9 cm in diameter at breast height within a radius of 2.82 m. Finally, within a 1-m radius from each plot’s centre, the understorey plants were identified.

Fig. 1. Map showing the six plots inventoried nearby the first observation of Aproceros leucopoda in Sainte-Martine, Québec, Canada. Plots were paired on both sides of the road, and a larger 200-m area was used to assess landscape use with satellite images.
The canopy coverage was measured using satellite images (Environmental Systems Research Institute 2021) in two zones for every plot: a small-scale zone covering exactly each of the six 5.64-m-radius plots and a larger-scale zone with a 200-m radius around each pair of plots that provided an idea of the surrounding landscape. The proportional coverage of three landscape types – forest, agricultural, and anthropogenic (i.e., roads and buildings) – was determined.
Elm zigzag sawfly damage
All elm trees within each 5.64-m-radius plot were examined for A. leucopoda damage. The entire canopy was examined, with the upper canopy being observed using binoculars. Damage was categorised depending on the degree of certainty that it was caused by A. leucopoda. Damage was characterised as A. leucopoda when zigzag defoliation was clearly visible. Indeed, early-instar larvae of some Sterictiphora species (Argidae, and also Sterictiphorinae such as Aproceros) are known to cause similar damage, but they all are restricted to Rosaceae for larval host plants (Eiseman Reference Eiseman2015; Eiseman and Smith Reference Eiseman and Smith2020). When defoliation was observed between veins but without any zigzag, this was considered consistent with damage by late-instar A. leucopoda and was recorded as probably A. leucopoda. Any other type of damage was ignored.
A Pearson correlation analysis was performed between the elm diameter at breast height and the total number of signs observed per tree using R software (R Core Team 2017).
Additional community science evidence
The Canadian Food Inspection Agency and the Canadian Forest Service posted requests on social media, asking communities to watch for any signs of this new insect (e.g., @MartelVronique3, 11 September 2020, https://twitter.com/MartelVronique3/status/1304526609731461120; @InspectionCan, 11 September 2020, https://twitter.com/InspectionCan/status/1304521361440886787). For any new report or observation, specimens were either collected by the observers and sent to the Canadian Food Inspection Agency, or Canadian Food Inspection Agency or Canadian Forest Service personnel visited the site to collect specimens for formal identification.
After the publication of a second observation on iNaturalist on 4 September 2020 (Drapeau-Picard Reference Drapeau-Picard2020), a search using iNaturalist’s custom URL feature was undertaken to find additional signs of the insect on elms. A custom search for elm observations centred on the midpoint of the two first feeding track records (45.6268, –73.4702), and the distance between them (108.84 km) was multiplied by 1.5 to define the search radius. Because it is possible that the species dispersed from one location to the other, we assumed that it could have done so equally in any direction and over the same distance. The search was restricted to verifiable observations recorded in the months of April–November (the probable active season), from April 2018 to May 2021. A total of 1281 elm observations were reviewed using iNaturalist’s identify function, at the following URL: https://inaturalist.ca/observations/identify?quality_grade=needs_id,research&lat=45.6268&lng=-73.4702&radius=163.26&year=2018,2019,2020&month=4,5,6,7,8,9,10,11&taxon_id=53549&place_id=any. Photos showing leaves in these observations were inspected for sign of A. leucopoda-feeding tracks.
A second custom search was performed to investigate the possibility of a separate introduction of A. leucopoda in Virginia, United States of America. This search was motivated by a June 2019 observation of an adult sawfly in Virginia (Seltzer Reference Seltzer2019), the resemblance to A. leucopoda of which was noted only after the species was first detected in Quebec in 2020. A custom URL search was used to review 1951 elm observations from within a 180-km radius of the Virginia observation, using the following URL: https://inaturalist.ca/observations/identify?quality_grade=needs_id,research&lat=39.037807&lng=-77.951755&radius=180&year=2018,2019,2020&month=4,5,6,7,8,9,10,11&taxon_id=53549&place_id=any.
Origin of the introduction
All specimens not sent for formal species identification by the Canadian Food Inspection Agency in Ottawa were stored in 70% ethanol for molecular-analysis comparisons with available European and Asian sequences. A total of 12 larvae were analysed: two larvae that were collected on 9 September 2020 in Saint-Pie, Québec (+45 178, –73 776) and 10 larvae that were collected on 11 September 2020 in Parc du Rocher, Saint-Amable, Québec (+45.645, –73.325).
DNA extraction
Samples of DNA were extracted from 12 individual larvae using the Qiagen DNeasy Blood and Tissue kit (cat. 69504; Qiagen, Hilden, Germany) following the “Purification of Total DNA from Animal Tissues” protocol provided with the kit. Larvae were crushed in a mix of 180 µL ATL buffer and 20 µL proteinase K, followed by an overnight incubation in a thermomixer (56 °C, 400 rpm 5 minutes/hour). The rest of the extraction followed the Qiagen protocol, including the facultative RNase step. The DNA was then eluted in 100 µL of water.
Mitochondrial cytochrome oxidase 1 barcode amplification and sequencing
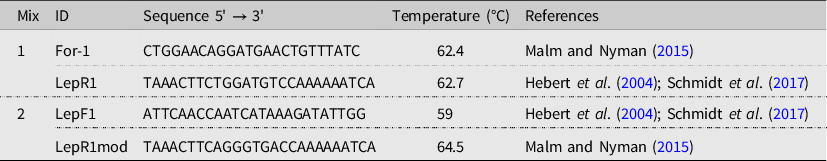
Polymerase chain reaction amplification of each DNA sample was conducted using Platinum SuperFi DNA Polymerase (Invitrogen; Thermo-Fisher Scientific Inc., Waltham, Massachusetts, United States of America), following the manufacturer’s instructions for genomic DNA (5–50 ng of DNA/reaction) and a three-step amplification protocol for fragments shorter than 10 kilobases in length. Amplification was done using CO1 forward and reverse primers, with two different sets of primers to obtain overlapping amplicons (Table 1), and under the following conditions: 30 seconds at 98 °C, plus 35 cycles at 98 °C for 10 seconds, at 58 °C for 10 seconds, and at 72 °C for 15 seconds, and a final extension step of 72 °C for 5 minutes. Amplicons were sequenced from both ends using the same forward and reverse primers used for polymerase chain reaction amplification (SANGER Sequencing platform, Centre hospitalier universitaire de Québec–Université Laval Research Centre, Québec City, Québec, Canada).
Table 1. Primer sequences used for polymerase chain reaction amplification in the present study.
Phylogenetic analysis
The unique sequence obtained for the Saint-Pie and Saint-Amable specimens (GenBank accession number: MW887550) was compared to six European and Asian A. leucopoda CO1 sequences of sufficient length found in GenBank: KC974833 (Austria-1), KF936623 (Austria-2), KC973566 (Romania), KC976874 (China), KC976060 (Russia-1), KC975690 (Russia-2; Malm and Nyman Reference Malm and Nyman2015; Schmidt et al. Reference Schmidt, Taeger, Morinière, Liston, Blank and Kramp2017) and to that of Aprosthema austriacum, HQ563827 (Germany) as an outgroup. Aprosthema austriacum belongs to the same Argid subfamily, Sterictiphorinae, as Aproceros (Schmidt et al. Reference Schmidt, Taeger, Morinière, Liston, Blank and Kramp2017). Analyses were carried out using the maximum-likelihood method and the Tamura-3 model (Tamura Reference Tamura1992) with 1000 bootstrap replicates (MEGA X; Kumar et al. Reference Kumar, Stecher, Li, Knyaz and Tamura2018), based on a MUSCLE alignment (Edgar Reference Edgar2004a, Reference Edgar2004b) of 658 shared nucleotide positions from the CO1 barcode region, with no gap.
Results
Initial observation and field mission
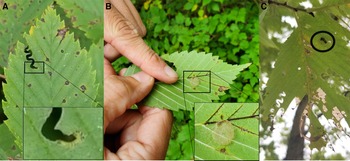
Four larvae (three small ones and a larger one) and one cocoon were collected in Sainte-Martine, Québec on 27 August 2020. Several signs of typical zigzag defoliation, larvae, empty cocoons, and one unemerged summer cocoon were discovered (Fig. 2). The larvae and the adult that emerged from the cocoon were formally identified as Aproceros leucopoda at the Canadian Food Inspection Agency entomology laboratory in Ottawa (CFIA#20-00981).
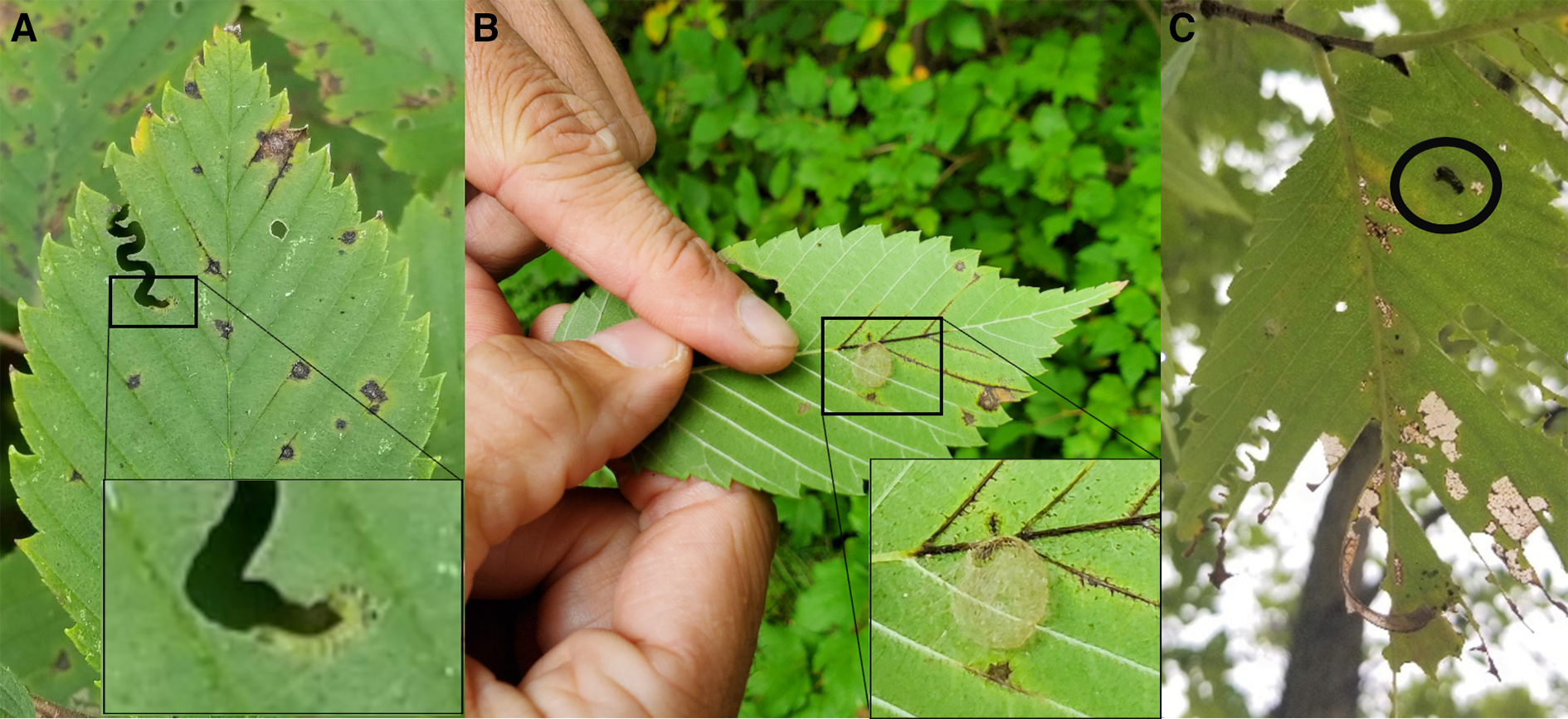
Fig. 2. A, Typical zigzag defoliation with a close-up on the feeding Aproceros leucopoda larva, B, an empty summer cocoon on an elm leaf, and C, an elm leaf with late-instar larval defoliation and an unemerged cocoon (circled), all taken on the first visited site in Sainte-Martine, Québec, Canada.
Characterisation of the first reported site
Site characteristics
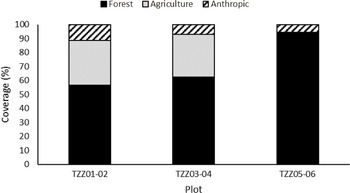
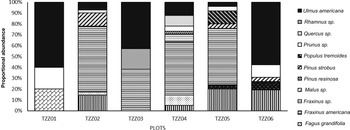
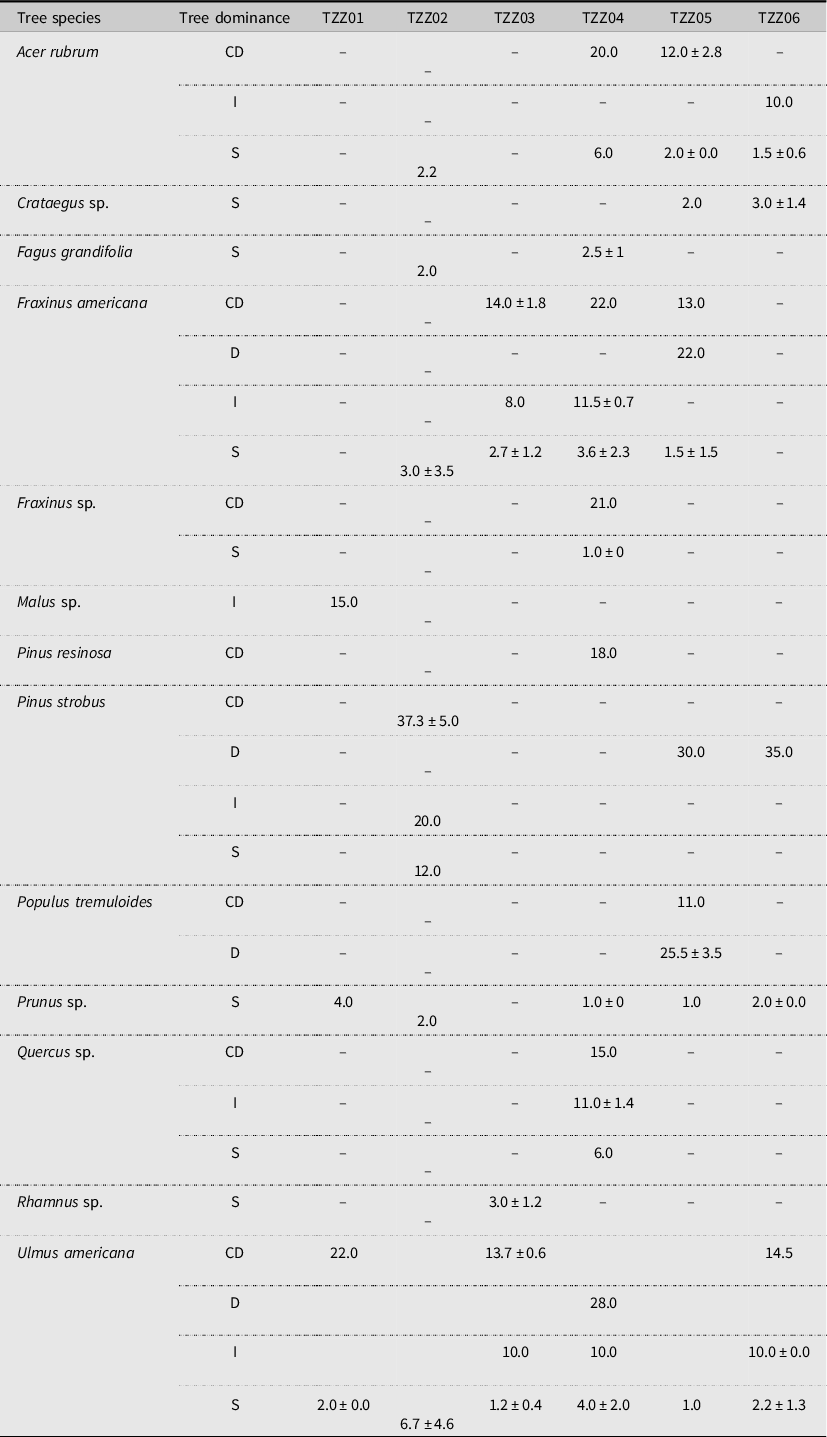
Analysis of satellite images showed that the landscape in the six sample plots consisted mainly of forests, although anthropogenic landscapes (roads and houses) and agricultural land were also present near four of the six paired plots (Fig. 3). Ulmus americana was the only elm species growing in the plots. A total of 37 elm trees were identified across all of the plots, accounting for up to 60% of the sites’ composition (Fig. 4), with an average diameter at breast height between 1.0 and 28.0 cm, depending on the plot (Table 2).
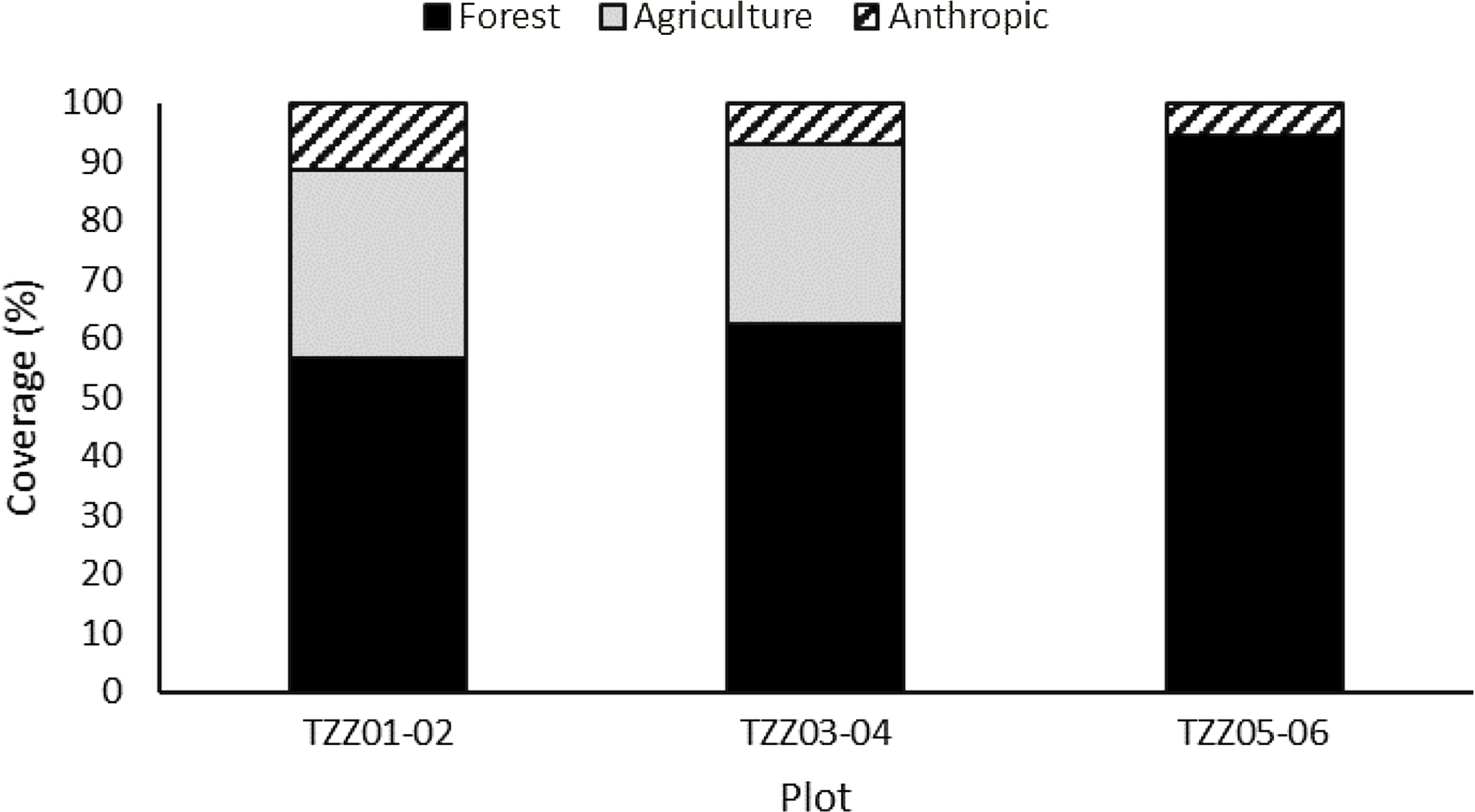
Fig. 3. Landscape usage in the 200-m radius around paired plots in Sainte-Martine, Québec, Canada obtained from the satellite image analysis.
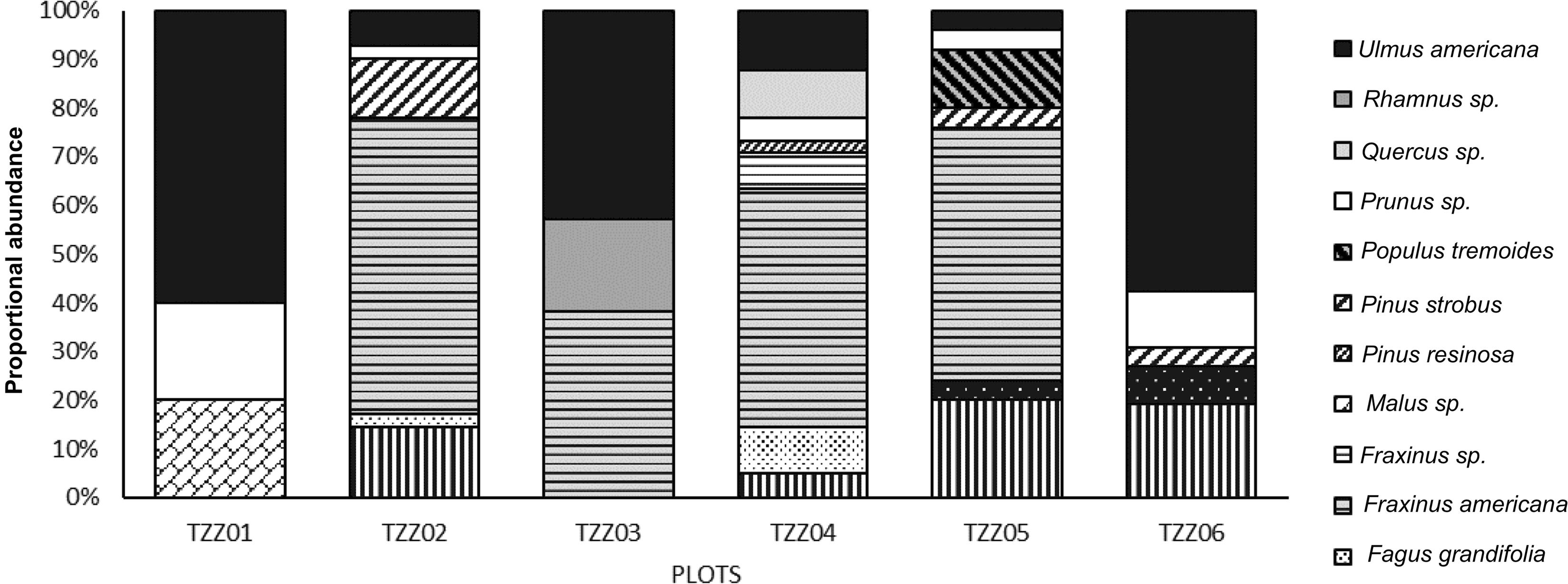
Fig. 4. Tree species composition for each plot in Sainte-Martine, Québec, Canada.
Table 2. Average diameter at breast height (± standard deviation, in cm) for each tree species depending on the dominance (D, dominant; CD, codominant; I, intermediate; S, suppressed) for each plot in Sainte-Martine, Québec, Canada. No standard deviation is given when only one tree of that species was inventoried.
Elm zigzag sawfly damage
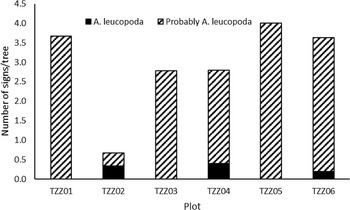
Damage was observed in every plot, on 27 of the 37 elm trees. For three of the six plots, as typical zigzag traces were not found, the damage was classified as probably from A. leucopoda (Fig. 5). A significant correlation occurred between the number of signs observed per tree and the tree’s diameter at breast height (r = 0.4656, t = 3.0681, df = 34, P = 0.004; Fig. 6).
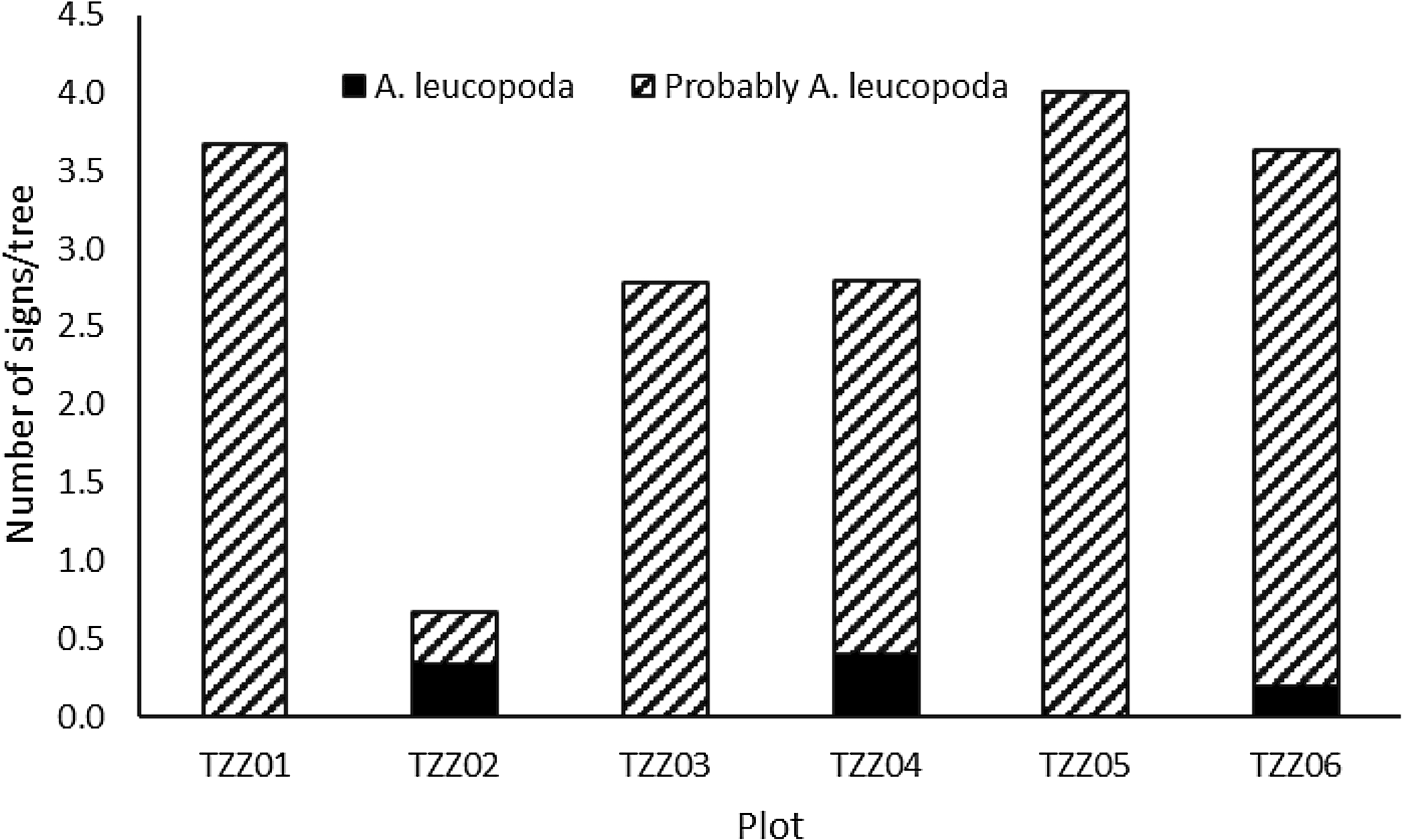
Fig. 5. Total number of signs observed depending on the number of elm trees in each plot, depending on the certainty that it was caused by Aproceros leucopoda.
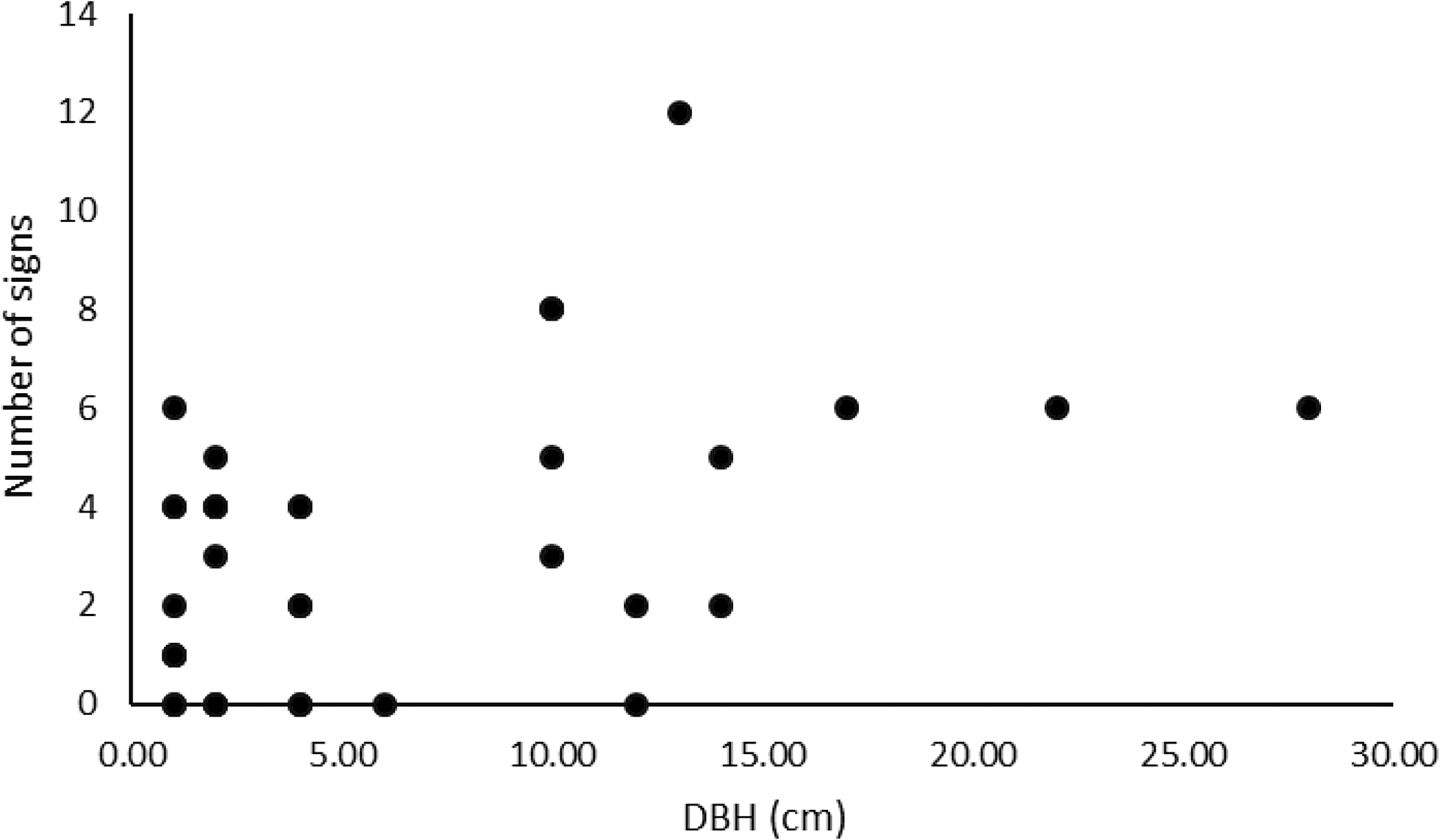
Fig. 6. Correlation between the number of signs observed on elm trees and their diameter at breast height (DBH) for all plots.
Additional community science evidence
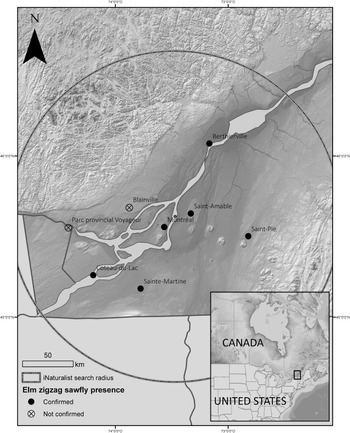
As a result of social media efforts to encourage observations, 12 iNaturalist observations showing signs of elm zigzag sawfly were recorded between 4 September and 7 November 2020 from the Montréal area, bringing the total observations to 15 (Table 3). The elm zigzag sawfly was confirmed through formal Canadian Food Inspection Agency identification at seven sites in six Québec municipalities (Fig. 7).
Table 3. Research-grade iNaturalist observations of larvae or feeding tracks of Aproceros leucopoda, recorded in Canada in 2020. ON, Ontario; QC, Québec.
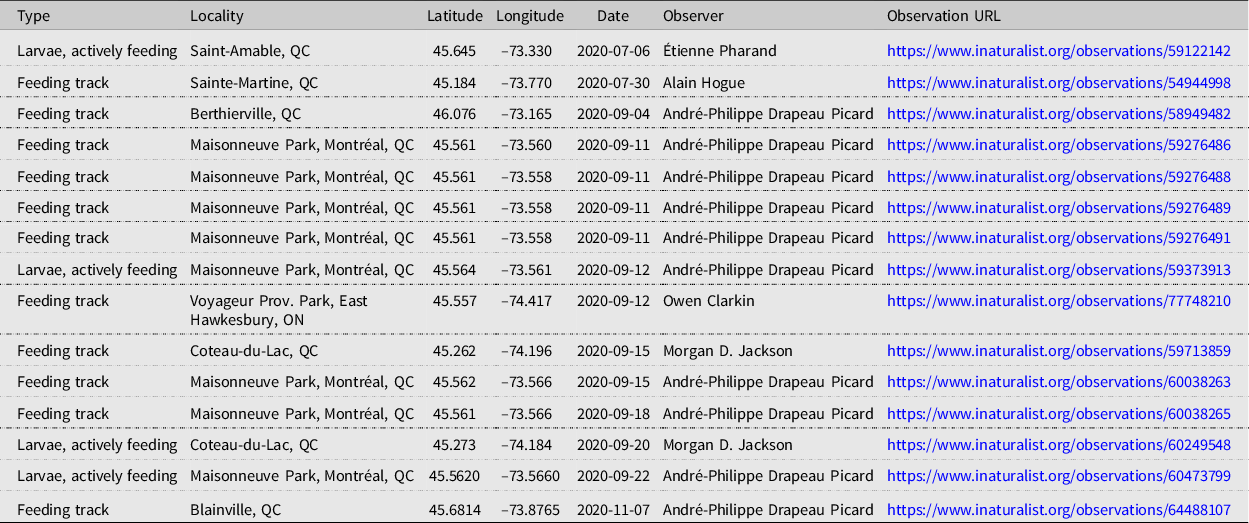

Fig. 7. Map showing the sites where the presence of Aproceros leucopoda was confirmed or suspected in Canada in 2020 and the area of the custom search on iNaturalist.
A search through 1281 iNaturalist elm observations yielded two that showed evidence of A. leucopoda. One previously overlooked observation was made on 6 July 2020, showing young larvae feeding along their characteristic zigzag tracks (Pharand Reference Pharand2020), and a visit to the site confirmed the presence of A. leucopoda through formal specimen identification. A second observation (made on 12 September 2020, but not posted to iNaturalist until May 2021) shows feeding tracks on an elm in Voyageur Provincial Park, Ontario (Clarkin Reference Clarkin2021), but the sawfly’s presence there had not been confirmed at the time of writing.
A secondary search of 1951 elm observations for the United States of America yielded a single observation from Virginia, made on 29 April 2019, that appears to show a zigzag feeding track with a larva present (McLean Reference McLean2019), although this could not be verified.
Origin of the introduction
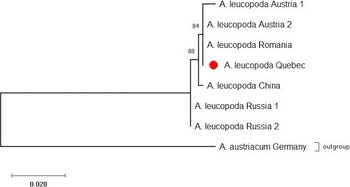
The CO1 barcode that was sequenced was identical for all 12 A. leucopoda larvae tested (GenBank accession number: MW887550). Comparison of this sequence with existing GenBank entries confirmed the insect’s identity as A. leucopoda. The CO1-based maximum-likelihood phylogenetic analysis placed the Québec specimens nearest those from Austria and Romania, supported by a relative high bootstrap value (84%) and pointing to a possible European origin of the Québec population (Fig. 8).
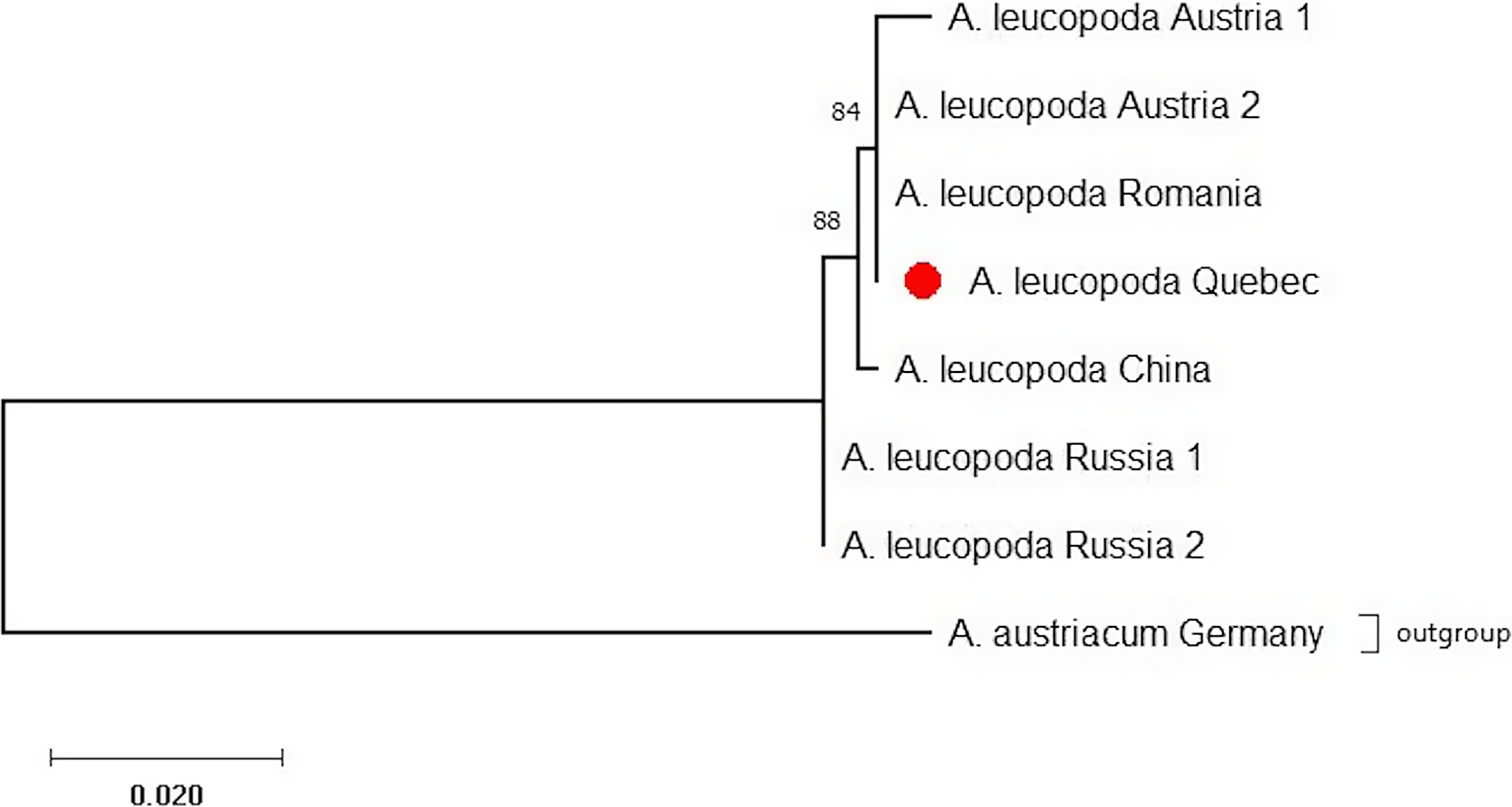
Fig. 8. Maximum-likelihood phylogenetic tree of CO1 barcode sequences from populations of Aproceros leucopoda in Québec, Europe, and Asia, generated using MUSCLE and MEGA X. Branch-length scale represents 0.02 substitution per site. See the Material and methods section for the GenBank accession numbers.
Discussion
This is the first record of the exotic species Aproceros leucopoda in North America and, more specifically, in Canada, and its detection was greatly facilitated by the community science website iNaturalist. The species has been confirmed in six municipalities in southern Québec, with additional observed signs to be confirmed in a seventh municipality in Québec, in eastern Ontario (one putative sign), and in Virginia (also one putative sign), United States of America, suggesting that the species probably arrived before 2020 – possibly in 2018 or 2019, based on estimates of Blank et al. (Reference Blank, Köhler, Pfannenstill, Neuenfeldt, Zimmer and Jansen2014) regarding the speed of self-dispersal. In this instance, community science and the use of social media proved to be useful not only in the detection of a new invasive species but also in assessing its distribution in Canada to date.
The forest inventory performed at the site of the first observation shows that the area is mainly forested and has both agricultural and anthropogenic landscape elements. This suggests that A. leucopoda could be present in forested as well as in urban habitats. Indeed, the iNaturalist observations are from a wide range of habitats, including from within the city of Montréal, in botanical gardens, and in urban parks. In Europe, A. leucopoda has been found in a variety of habitats as well: along motorways, in parking areas, in cities, in botanical gardens and arboreta, in woods, and on mountains – everywhere elms grow – but it has been suggested that solitary elms in urban areas and recently planted trees may be more likely to be attacked (Zandigiacomo et al. Reference Zandigiacomo, Cargnus and Villani2011; Blank et al. Reference Blank, Köhler, Pfannenstill, Neuenfeldt, Zimmer and Jansen2014; Mol and Vonk Reference Mol and Vonk2015; Holuša et al. Reference Holuša, Stodůlková and Macek2017; Vétek et al. Reference Vétek, Bartha and Oláh2017). In the Netherlands, 71% of observations of A. leucopoda were within or near urban areas, and 32% were near roads and railways (Mol and Vonk Reference Mol and Vonk2015). This makes A. leucopoda an even better candidate for detection through community science.
In the present study, damage was observed on 73% of all elm trees in the sample plots and on trees of all sizes, although more signs were observed on larger-diameter trees. The damage was observed on U. americana, the only elm species found to be growing in the plots. However, A. leucopoda has been found to attack many native and introduced elm species in Europe, including cultivars resistant to Dutch elm disease (Zandigiacomo et al. Reference Zandigiacomo, Cargnus and Villani2011; Blank et al. Reference Blank, Köhler, Pfannenstill, Neuenfeldt, Zimmer and Jansen2014; Vétek et al. Reference Vétek, Bartha and Oláh2017), and at least some of the damaged elms observed in Québec via iNaturalist appear to be U. rubra. It should therefore be assumed that all elm species in North America are potential hosts for this exotic defoliator. Because the formal identification of the species occurred late in the season (early September), further studies covering the entire growing season will be undertaken to assess the species’ exact phenology in this new habitat, including the number of generations it can undergo under the local climate, the elm species it attacks or prefers, its potential impacts, and other basic life-history traits. In particular, its impact on elm varieties resistant to Dutch elm disease will need to be investigated to assess its risk to elm populations in Canada.
This study highlights the importance of community science in the detection of exotic species. Although community science is far from new, it has only grown in visibility and popularity, thanks in part to apps and websites like iNaturalist, which recorded its 50-millionth observation in September 2020 – approximately twice as many as had been recorded only a year earlier (iNaturalist 2020). Observations such as those reported on iNaturalist offer great potential for detecting new pest threats. For example, the platform Mosquito Alert allowed for the first detection of an exotic mosquito, Aedes japonicus (Theobald) (Diptera: Culicidae), in Spain (Eritja et al. Reference Eritja, Ruiz-Arrondo, Delacour-Estrella, Schaffner, Álvarez-Chachero and Bengoa2019), and iNaturalist facilitated the detection of the European firebug, Pyrrhocoris apterus Linnaeus (Hemiptera: Pyrrhocoridae), in Ontario (Oviedo Rojas and Jackson Reference Oviedo Rojas and Jackson2018). Similarly, the first detection of a North American fish, Cynoscion regalis (Bloch & Schneider) (Pisces: Sciaenidae), in Europe was made by fishermen in Portugal and reported in specialised magazines, personal blogs, and regional media (Morais and Teodósio Reference Morais and Teodósio2016). Although data collected through community science projects are sometimes criticised – for example, for their lack of true absence data, their quality, or their bias in geographic coverage – reported observations can often be validated, thereby making valuable contributions to science while also decreasing the costs of research and increasing community awareness (Crall et al. Reference Crall, Newmann, Jarnevich, Stohlgren, Waller and Graham2010; Bois et al. Reference Bois, Silander and Mehrhoff2011; Johnson et al. Reference Johnson, Mader, Dasgupta and Jumar2020; Larson et al. Reference Larson, Graham, Achury, Coon, Daniels and Gambrell2020; Encarnação et al. Reference Encarnação, Teodósio and Morais2021).
Community science apps may furthermore hold potential for monitoring for invasive species using methods similar to those outlined in the present study. This potential was in fact explored in the course of the present study: besides yielding the earliest observation of A. leucopoda in Québec, the iNaturalist custom URL search feature was also used to uncover the first suggested evidence of this species in Ontario and to investigate the possibility of a separate introduction in Virginia, United States of America. In the latter case, the putative observations from Virginia of an adult sawfly (Seltzer Reference Seltzer2019) and larva (McLean Reference McLean2019) – although not verified – have helped prompt on-the-ground monitoring in several other states.
The preliminary data on the genetic comparison of the DNA barcode sequences from Canadian specimens of A. leucopoda with the sequences of European and Asian specimens available from GenBank suggest the Canadian specimens derive from a European origin, with the sequences from Austria and Romania grouping with those from Québec. However, these results must be interpreted with caution, as only 12 specimens from Canada were sequenced and the number of European and Asian sequences available for comparison is also limited. Additional molecular investigations involving multiple markers will be conducted in 2021 with additional specimens and sequences from Canada, Europe, and Asia to more precisely identify the geographic origin of this introduction. Molecular analysis could eventually help to determine if one or more introductions occurred. The absence of variability among specimens of our small, geographically restricted sample might indicate that the Canadian population is based on the introduction and successful establishment of a single female of A. leucopoda, which reproduced parthenogenetically; this observation requires closer scrutiny.
The exact entrance pathway of this new invasive species to Canada remains unknown, even with the suggestion of a European origin. However, we speculate that it was introduced in the winter cocoon stage. Indeed, any other life stage would likely have died during transportation due to the combination of quick development and a lack of food (imported trees are transported in a dormant stage without foliage). Winter cocoons are formed on the ground and may have been transported within soil. Canadian import requirements prohibit entry of items with soil from off-continent, but fortuitous introductions of soil-borne pests are possible. The area where the Québec population was found may not be the point of introduction of the population; the insect may have dispersed from another locality instead.
Acknowledgements
The authors thank Karen McLachlan Hamilton and Graham Thurston from the Canadian Food Inspection Agency Entomology Lab for insect identification. They are also grateful to the individuals who reported defoliation or collected specimens: Alain Hogue, André-Philippe Drapeau Picard, Étienne Pharand, Morgan D. Jackson, Simon Amyot, Owen Clarkin, Carrie Seltzer, and Mary D. McLean. They appreciate the support of James Pagé from the Canadian Wildlife Federation for encouraging observations on iNaturalist. Finally, for their help in the field, in the lab, with analyses, and for comments on the manuscript, the authors thank Simon Trudeau, Sébastien Bélanger, Jessica Girona, Josée Laroche, Abdelmadjid Djoumad, and Erin Bullas.
This paper is dedicated to the memory of Dr. Gábor Vétek, who passed away too soon in December 2020.