Impact statement
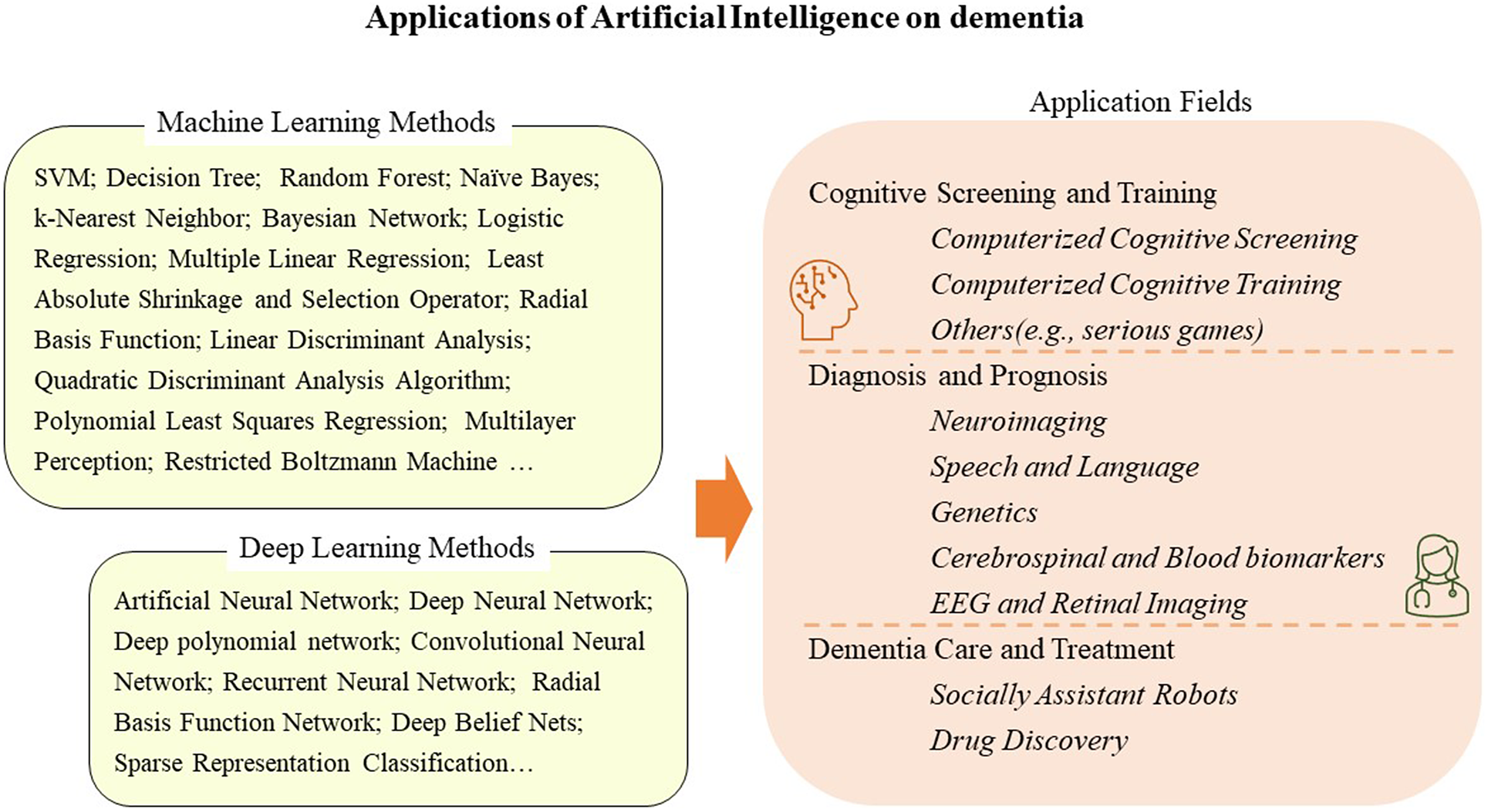
Artificial intelligence becomes popular for dementia research. Supervised and unsupervised machine learning models can be applied for cognitive screening, diagnosis, prognosis, and potentially for dementia care and treatment development.
Introduction
Dementia is a syndrome characterized by deterioration of cognitive function and behavior beyond what might be expected from the usual consequences of biological aging (Ernst and Hay, Reference Ernst and Hay1994; Bouchard, Reference Bouchard2007). The prevalence rate among those aged ≥60 years in different world regions is approximately 5%–7% (Prince et al., Reference Prince, Bryce, Albanese, Wimo, Ribeiro and Ferri2013), and the total number of dementia cases is expected to increase from 57.4 million in 2019 to approximately 150 million by 2050 (Nichols et al., Reference Nichols, Steinmetz, Vollset, Fukutaki, Chalek, Abd-Allah and Vos2022). The etiological subtypes of dementia include Alzheimer’s disease (AD), vascular dementia, frontotemporal dementia (FTD), frontotemporal lobar dementia, Huntington’s disease, Lewy bodies, and Parkinson’s disease (Bouchard, Reference Bouchard2007). Mild cognitive impairment (MCI) is also regarded as an early stage of dementia (Petersen, Reference Petersen2004; Morris, Reference Morris2006). AD accounts for approximately 60%–70% of diagnosed dementia cases and attracts the most attention from researchers. Early detection and timely diagnosis are challenging as the diagnoses of dementia are based on a comprehensive procedure of semi-structured interviews, cognitive tests, and medical examinations, which are time-consuming, costly, and sometimes even invasive. AD occurs mainly in the elderly, and it is difficult to distinguish between a degenerative condition and the general impact of aging. Approximately 29%–76% of individuals with dementia are unrecognized in clinical practice (Valcour et al., Reference Valcour, Masaki, Curb and Blanchette2000; Knopman et al., Reference Knopman, Boeve and Petersen2003; Chodosh et al., Reference Chodosh, Petitti, Elliott, Hays, Crooks, Reuben and Wenger2004).
Researchers employed artificial intelligence (AI) in clinical decision support systems, new therapy discovery, and genomics research by using different biomarkers of dementia (Miao et al., Reference Miao, Jiang, Liu and Yao2017; Zhu et al., Reference Zhu, Li, Tang, He, Zhang, Hung and Zhou2020; Anastasio, Reference Anastasio2021). Those biomarkers are measurable indicators of a biological state for dementia or cognitive decline, including neuroimaging, retinal imaging, language information, cerebrospinal and blood biomarkers, and gene information. AI has led to great breakthroughs in image processing (Krizhevsky et al., Reference Krizhevsky, Sutskever and Hinton2012) and natural language processing (NLP), such as the development of speech-to-speech translation engines and spoken dialogue systems (Hirschberg and Manning, Reference Hirschberg and Manning2006) that allow for more complicated and fast analysis of neuroimaging and speech data. For complex data sources such as magnetic resonance imaging (MRI), position emission tomography (PET) neuroimaging, and cerebrospinal fluid (CSF) biomarkers (Suk et al., Reference Suk, Lee and Shen2016; Ebrahimighahnavieh et al., Reference Ebrahimighahnavieh, Luo and Chiong2020), deep learning methods (e.g., neural network-related methods) can be applied to build diagnostic classifiers or applied in feature extraction steps (e.g., Auto-Encoder; Ebrahimighahnavieh et al., Reference Ebrahimighahnavieh, Luo and Chiong2020). The support vector machine (SVM) algorithm is the most widely used machine learning method to classify diseases like Alzheimer’s, Epilepsy, and Parkinson’s (Deepa et al., Reference Deepa, Shanmugam and Tamilselvan2017). The neural network-based methods are also popular, including multilayer perception and deep learning methods such as the convolutional neural network (CNN). An upsurge in chemical data availability makes AI viable for virtual screening (VS) of drug discovery (Vamathevan et al., Reference Vamathevan, Clark, Czodrowski, Dunham, Ferran, Lee and Zhao2019). The global popularity of smartphones makes it possible to deploy a variety of mobile apps for cognitive training and screening, including traditional cognitive tests and unconventional new methods (Thabtah et al., Reference Thabtah, Peebles, Retzler and Hathurusingha2020; Chelberg et al., Reference Chelberg, Neuhaus, Mothershaw, Mahoney and Caffery2021). Social robots were developed to assist dementia patients in performing basic instrumental activities of daily living (Schroeter et al., Reference Schroeter, Mueller, Volkhardt, Einhorn, Huijnen, van and Gross2013; Law et al., Reference Law, Sutherland, Ahn, MacDonald, Peri, Johanson and Broadbent2019; Ghafurian et al., Reference Ghafurian, Hoey and Dautenhahn2021).
This review summarizes AI in dementia research and its application to (i) dementia diagnosis and prognosis, (ii) cognitive screening and training, and (iii) care and treatment. The paper search was conducted on Ovid Embase, MEDLINE, Web of Science, IEEE Xplore, and ScienceDirect with the following keywords: artificial intelligence or machine learning or deep learning or support vector machine or decision tree and dementia or cognitive or Alzheimer. Papers that applied machine learning technology on dementia were included whether they were original study or reviews.
Basic concepts of artificial intelligence
AI is a general term that means imitating intelligent human behavior using a computer with minimal human intervention. Research into AI applications began shortly after the official naming of AI at a Dartmouth College meeting in 1956 (Mishra and Li, Reference Mishra and Li2020). Machine learning is a subfield of AI that works by examining and learning patterns of input datasets to build models for classification, regression, and clustering. Deep learning, reinforcement learning, and transfer learning are more specific subsets of machine learning. As reported in Table 1, machine learning methods include SVM, random forest (RF), k-nearest neighbor (k-NN), and so on. Deep learning methods are neural network-based methods, such as CNN and artificial neural network (ANN). It has only recently become a trend with the onset of the “Big Data” era, although it has existed since the 1950s.
Table 1. Abbreviation for machine learning methods*

* The list only shows machine learning methods reported in this study.
Machine learning can be divided into three types: supervised, unsupervised, and semi-supervised learning, according to whether the input data are labeled (Kumar et al., Reference Kumar, Oh, Schindler, Lai, Payne and Gupta2021). Supervised learning means training a model on a dataset annotated with labels applied in classification and regression tasks, such as linear regression (LR) and SVM. In comparison, unsupervised models learn from unlabeled data by extracting features and patterns in solving clustering problems, such as k-NN and principal component analysis. Finally, the semi-supervised method builds a model on a training dataset with labels in one part and no labels in the other. A specific algorithm does not necessarily belong to only one of the three types. For example, semi-supervised SVM is a good solution when the datasets contain unlabeled data, whereas the standard SVM cannot perform well in this situation (Ding et al., Reference Ding, Zhu and Zhang2017).
Applications in dementia diagnosis and prognosis
The complex process of dementia diagnosis involves a combination of medical examinations and professional clinicians. The Diagnostic and Statistical Manual of Mental Disorders (DSM) is widely used as a general diagnostic criterion for dementia, and the most updated version is DSM-5 (Bouchard, Reference Bouchard2007; American Psychiatric Association, 2013). However, it does not represent all the clinical profiles of some subtypes of dementia, such as vascular and FTD, where memory impairment is not necessarily the first requirement. Currently, there are also other different diagnostic criteria which are used in clinical settings, and dementia research (e.g., NINCDS-ADRDA10 criteria and 10th revision of the International Classification of Diseases; McKhann et al., Reference McKhann, Folstein, Katzman, Price and Stadlan1984; World Health Organization, 1992; Bouchard, Reference Bouchard2007). Cognitive deficits might appear in many other diseases, but only those diseases whose core features are cognitive disorders and decline are included as neurocognitive disorders. Diagnosis involves cognitive function, language, praxis, gnosis, executive function, and other medical tests. Overall, there are no perfect criteria, and the diagnostic process is complicated. A large number of patients remain undiagnosed due to costly and time-consuming procedures (Prince et al., Reference Prince, Comas-Herrera, Knapp, Guerchet and Karagiannidou2016). The disease progresses, before symptoms appear clearly, while patients experience mild-to-moderate cognitive impairment.
Machine learning methods are widely applied in high-dimensional clinical data for dementia prediction (Spooner et al., Reference Spooner, Chen, Sowmya, Sachdev, Kochan, Trollor and Brodaty2020). The Alzheimer’s Disease Prediction of Longitudinal Evolution Challenge was held in 2017, which aims to identify the most effective features and approaches that predict clinical diagnosis of AD, Alzheimer’s Disease Assessment Scale Cognitive Subdomain (ADAS-Cog13), and total volume of the ventricles (Marinescu et al., Reference Marinescu, Oxtoby, Young, Bron, Toga, Weiner and Alexander2021). The challenge compared the performance of 92 algorithms and found that those algorithms perform differently on three outcomes, and CSF samples and diffusion tensor imaging were associated with better diagnosis performance (Marinescu et al., Reference Marinescu, Oxtoby, Young, Bron, Toga, Weiner and Alexander2021). With the number of publications increasing drastically since 2017 (Ebrahimighahnavieh et al., Reference Ebrahimighahnavieh, Luo and Chiong2020), deep learning has begun to gain considerable attention in research for AD detection. The data sources applied in AI models are beyond the traditional data format, such as age, gender, and comorbidity. New forms of cognitive data include neuroimaging, speech and language, genetic research, CSF and blood biomarkers, and electroencephalogram (EEG) and retinal imaging (Figure 1).

Figure 1. Applications of AI on Dementia Diagnosis and Prognosis
Neuroimaging
Specific changes in brain structure and other metabolite responses in the brain can be measured by modern techniques, such as PET and MRI. Machine learning methods have been developed to detect disease via neuroimaging with improved medical imaging and greater availability of neuroimaging data (Pellegrini et al., Reference Pellegrini, Ballerini, Hernandez, Chappell, Gonzalez-Castro, Anblagan and Wardlaw2018). Due to the complexity of imaging data, feature extraction for neuroimaging data is still a challenge for data scientists. Feature extraction of imaging data can be generally grouped into four categories: voxel-based, slice-based, patch-based, and regions-of-interest (ROIs)-based features (Ebrahimighahnavieh et al., Reference Ebrahimighahnavieh, Luo and Chiong2020). ROI-based and patch-based methods were reported to be better pre-processing methods as they can exclusively include AD-related features in neuroimaging (Ebrahimighahnavieh et al., Reference Ebrahimighahnavieh, Luo and Chiong2020).
The advanced processing power of Graphics Processing Units makes it possible to apply deep learning methods in neuroimaging, particularly for CNNs, showing good performance in detecting disease by medical imaging (Ebrahimighahnavieh et al., Reference Ebrahimighahnavieh, Luo and Chiong2020). Pellegrini et al. (Reference Pellegrini, Ballerini, Hernandez, Chappell, Gonzalez-Castro, Anblagan and Wardlaw2018) reviewed studies from 2006 to 2016 and eventually included 111 studies of machine learning of neuroimaging on dementia detection. More than half of those studies applied SVM, and other methods, such as adaptive boosting (AdaBoost), linear discriminant analysis, and RF, were also involved. In the recent decade, deep learning has been a trend in the neuroimaging data processing. It imitates the working of the human brain and can merge complicated feature extraction and classification in solving complex problems. A review focused on deep learning and neuroimaging included more than 100 papers, all of which were conducted after 2013 and most (80%) after 2017 (Ebrahimighahnavieh et al., Reference Ebrahimighahnavieh, Luo and Chiong2020). Hidden Markov Model, one of the reinforcement learning methods, is especially suitable for detecting the progression of dementia by analyzing sequence neuroimaging data, and it has been applied by different research groups (Chen and Pham, Reference Chen and Pham2013; Williams et al., Reference Williams, Storlie, Therneau, Clifford and Hannig2019). Most studies pay closer attention to technical details, but have less of a clinical focus (Pellegrini et al., Reference Pellegrini, Ballerini, Hernandez, Chappell, Gonzalez-Castro, Anblagan and Wardlaw2018). A clinical-based framework is, therefore, needed to improve the application of the AI models.
Speech and language
Recent studies have suggested that language dysfunction is one of the earliest signs of cognitive disorders and a possible biomarker for the early detection of dementia (Ahmed et al., Reference Ahmed, Haigh, de Jager and Garrard2013; Mueller et al., Reference Mueller, Koscik, Hermann, Johnson and Turkstra2017; Beltrami et al., Reference Beltrami, Gagliardi, Rossini Favretti, Ghidoni, Tamburini and Calza2018; Garcia et al., Reference Garcia, Ritchie and Luz2020). Speech and language have long been used as important clinical information for dementia diagnosis, such as the Boston naming test studied and reported since 1986 (Knesevich et al., Reference Knesevich, LaBarge and Edwards1986). It can be obtained through content-based (specific tasks) (Rodriguez-Aranda et al., Reference Rodriguez-Aranda, Waterloo, Johnsen, Eldevik, Sparr, Wikran and Vangberg2016; Venneri et al., Reference Venneri, Jahn-Carta, De Marco, Quaranta and Marra2018) or content-free approaches (spontaneous conversation) (Huff, Reference Huff1986; Becker et al., Reference Becker, Boller, Lopez, Saxton and McGonigle1994). The dysfunction includes word retrieval difficulties (e.g., verbal naming, accurate meaning communication, and pulsation) and a tendency to repeat words or sentences. NLP plays an essential role in speech and text data analysis to extract prosodic, acoustic, or other features in dementia analysis (Jaffe and Feldstein, Reference Jaffe and Feldstein1970; Forbes et al., Reference Forbes, Venneri and Shanks2002; Weiner et al., Reference Weiner, Engelbart and Schultz2017; Luz et al., Reference Luz, de la Fuente and Albert2018). Researchers widely transformed NLP in the 1990s to build models over large quantities of empirical language data (Hirschberg and Manning, Reference Hirschberg and Manning2006). Traditional text feature extraction includes filtration, fusion, mapping, and clustering, which result in a lengthy process. Deep learning can be used to quickly acquire effective characteristics from training data, and CNN and recurrent neural networks (RNN) are two popular models (Liang et al., Reference Liang, Sun, Sun and Gao2017). Early NLP on the text-based dialogue has expanded to include spoken dialogue with the development of spoken dialogue systems and automatic speech recognition, which allows a more effective speech feature extraction (Weiner et al., Reference Weiner, Engelbart and Schultz2017).
Although many studies were conducted in building diagnostic models with a series of machine learning methods, such as SVM, decision tree, and RF, the expansion of those models is limited due to the small sample size and incomparable datasets. In 2020, the Alzheimer’s Dementia Recognition through Spontaneous Speech Challenge at INTERSPEECH 2020 provided an opportunity to use all available audio and textual data from a benchmark speech dataset. The challenge defined shared tasks and provided a standardized dataset based on spontaneous speech and allowed different research groups to test the performance of their existing or novel methods (Luz et al., Reference Luz, Haider, de la Fuente, Fromm and MacWhinney2020).
Genetics
Approximately 58%–79% of the risk of late-onset Alzheimer’s is heritable (Gatz et al., Reference Gatz, Reynolds, Fratiglioni, Johansson, Mortimer, Berg and Pedersen2006; Sims et al., Reference Sims, Hill and Williams2020). Studies suggest that patients with FTD (Greaves and Rohrer, Reference Greaves and Rohrer2019) or Lewy bodies dementia (Guerreiro et al., Reference Guerreiro, Escott-Price, Hernandez, Kun-Rodrigues, Ross, Orme and Bras2019) have a high proportion of positive family history in as many as 60% of cases (Mackenzie et al., Reference Mackenzie, Frick and Neumann2014). High-dimensional genetic-related data have been generated with the evolution of genomics. AI methods, such as SVM, have shown good performance on gene identification and pathway analysis, and RF is well suited for microarray data (Miao et al., Reference Miao, Jiang, Liu and Yao2017). Xu et al. (Reference Xu, Liang, Liao, Chen and Chang2018) applied the SVM model to predict AD by using protein sequence, and the accuracy rate was 85.7%, where RF, naïve Bayes, AdaBoost, and Bayes network were also applied and compared with the result of SVM. Varatharajah et al. (Reference Varatharajah, Ramanan, Iyer and Vemuri2019) integrated genetic data, multimodal neuroimaging, CSF biomarkers, genetic factors, and measures of cognitive resilience data to build an SVM model to predict the progress of MCI to AD within 3 years with an accuracy rate up to 93%. Machine learning methods can also be applied in detecting significant genetic variants, gene expression, and gene–gene interaction (Mishra and Li, Reference Mishra and Li2020).
Cerebrospinal fluid and blood biomarkers
Measuring Amyloid-Beta, total tau (T-tau), and hyperphosphorylated tau (p-tau) in CSF proteins has proved accurate in diagnosing AD (Ritchie et al., Reference Ritchie, Smailagic, Noel-Storr, Ukoumunne, Ladds and Martin2017; Zetterberg, Reference Zetterberg2019). However, these biomarkers are expensive and relatively invasive. In addition to invasive methods, imaging technology can reflect the level of CSF biomarkers (e.g., tau PET). With the recent advent of highly sensitive and specific immune and mass spectrometry-based assays, CSF biomarkers can also be detected through blood (Varma et al., Reference Varma, Oommen, Varma, Casanova, An, rews and Thambisetty2018). Other blood biomarkers include N-methyl-D-aspartate receptor-mediated biomarkers and metabolites biomarkers. The first d-glutamate-based study that applied machine learning models to detect MCI and AD in healthy people was published in 2021 (Chang et al., Reference Chang, Lin, Liu, Huang, Chen, Lin and Lane2021). A total of 133 AD patients, 21 MCI patients, and 31 healthy controls were recruited, and four machine learning algorithms (SVM, LR, RF, and Naïve Bayes) were employed to build predictive models to distinguish MCI or AD patients from healthy controls, with sex, age, and d-glutamate as predictors. The Naïve model and the RF model showed the best performance with area under the curve (AUC) of 0.82 and 0.79 (Chang et al., Reference Chang, Lin, Liu, Huang, Chen, Lin and Lane2021). Stamate et al. (Reference Stamate, Kim, Proitsi, Westwood, Baird, Nevado-Holgado and Legido-Quigley2019) applied deep learning, extreme gradient boosting, and RF on plasma metabolites data to differentiate healthy people and patients with AD. They also showed better AUC than the results from amyloid, p-tau, and t-tau. Although the AUC seems good, which is usually more than 0.80, most studies lack external validation. Further studies are needed to assess the performance of combinations of CSF, blood biomarkers, and lifestyle factors.
Electroencephalogram and retinal imaging
An EEG is a test, administered by hospital equipment and wearable devices, that detects abnormalities in brain waves or in the electrical activity of the brain. In the recent decade, researchers have set their sights on AD diagnosis and progression based on EEG data ((Malek et al., Reference Malek, Baker, Mann and Greene2017; Stancin et al., Reference Stancin, Cifrek and Jovic2021). Due to the complexity of EEG data, feature extraction is a crucial step, including time-domain and frequency-domain features, nonlinear features, entropies, spatiotemporal features, and complex networks (Stancin et al., Reference Stancin, Cifrek and Jovic2021). Each feature contains a series of the index and is explored by many studies (Deepa et al., Reference Deepa, Shanmugam and Tamilselvan2017; Jaya Shree and Venkateshwarlu, Reference Jaya Shree and Venkateshwarlu2021). SVM is widely used for binary classification (Staudinger and Polikar, Reference Staudinger and Polikar2011; Jaya Shree and Venkateshwarlu, Reference Jaya Shree and Venkateshwarlu2021; Tzimourta et al., Reference Tzimourta, Christou, Tzallas, Giannakeas, Astrakas, Angelidis and Tsipouras2021). Sharma et al. (Reference Sharma, Kolekar and Jha2021) conducted a multiclass SVM in 2021 with a diagnostic accuracy of 87.6%, in which they initially extracted 12 EEG features and then selected five of them through analysis of variance. Deep learning methods, such as RNN and ANN, are rapidly increasing in EEG studies. Ieracitano et al. (Reference Ieracitano, Mammone, Hussain and Morabito2020) proposed a multimodal machine learning that integrated Multilayer Perceptron, LR, and SVM to classify MCI and dementia using EEG data.
Retinal imaging is a cost-effective replacement for neuroimaging as retinal changes can reflect the pathology of the brain. The quantitative analysis of vessel calibers, tortuosity, and network complexity in retinal imaging data provide diagnostic value for dementia. Tian et al. (Reference Tian, Smith, Guo, Liu, Pan, Wang and Fang2021) proposed a multistage pipeline that involved SVM and CNN and achieved an average diagnostic accuracy of 82.4% for AD.
Applications in cognitive screening and training
Several mobile apps have been developed to screen normal individuals’ cognitive function before they suffer from MCI or dementia (Thabtah et al., Reference Thabtah, Peebles, Retzler and Hathurusingha2020). Most mobile apps assist individuals diagnosed with MCI or dementia in brain training. In addition to apps, machine learning can also contribute to building new assessments for MCI or dementia. Chiu et al. (Reference Chiu, Tang, Wei, Zhang, Hung and Zhou2019) applied information gain, which is a feature selection method in RF, in developing a brief questionnaire to help clinicians in dementia diagnosis. With advances in wearable technologies, plenty of data collected from wearable sensors can also be applied in machine learning models and thus improve the performance (Iaboni et al., Reference Iaboni, Spasojevic, Newman, Schindel, Wang, Ye and Khan2022).
Computerized cognitive screening
Dementia screening aims to identify those in the prodromal phase of dementia by using neuropsychological tests (Panegyres et al., Reference Panegyres, Berry and Burchell2016). The tests include the Abbreviated Mental Test, the Montreal Cognitive Assessment (MoCA), the Mini-Mental State Examination (MMSE), and others. Several dementia screening methods are now available on mobile apps, making them more accessible to patients, caregivers, and medical staff. Those screening tools could be divided into three categories (Thabtah et al., Reference Thabtah, Peebles, Retzler and Hathurusingha2020): (1) apps based on a single medical assessment method (such as MMSE and MoCA), (2) apps based on multiple medical assessment methods (e.g., DementiaTest, which integrated six-item cognitive impairment and the structured clinical interview; Thabtah et al., Reference Thabtah, Mampusti, Peebles, Herradura and Varghese2019), and (3) apps based on nonconventional methods. Cognity is one of the eligible apps, which applied AI technology to screen for AD by combining analysis of a clock photo drawing by the user and the Mental Status Examination (Thabtah et al., Reference Thabtah, Peebles, Retzler and Hathurusingha2020).
A systematic review in 2020 evaluated mobile apps of dementia screening available on Android and Apple platforms (Thabtah et al., Reference Thabtah, Peebles, Retzler and Hathurusingha2020). The evaluated criteria were based on DSM-5, including six domains of cognitive function. They initially found 275 apps in the English language, and only 20 apps were eligible. Most excluded apps were games and informative apps to assist individuals in their cognitive functions and skills (Thabtah et al., Reference Thabtah, Peebles, Retzler and Hathurusingha2020). Another systematic review performed by Chan et al. (Reference Chan, Yau, Kwok and Tsoi2021) evaluated the diagnostic performance of digital tests, finding a few validation studies for all digital tests, and the eligible apps had a sensitivity and specificity of more than 0.8.
Computerized cognitive training
Cognitive training via digital devices is a promising strategy for maintaining the cognitive function of healthy elderly and MCI patients (Zhang et al., Reference Zhang, Huntley, Bhome, Holmes, Cahill, Gould and Howard2019). The main advantages are the active accessibility and timely feedback (Irazoki et al., Reference Irazoki, Contreras-Somoza, Toribio-Guzman, Jenaro-Rio, van and Franco-Martin2020). In addition to providing cognitive training for normal people or MCI patients, most training apps focus on caregivers or family members of dementia patients to assist them in caring for dementia patients. A review performed by Chelberg et al. (Reference Chelberg, Neuhaus, Mothershaw, Mahoney and Caffery2021) included 75 Australian-based apps, focusing on cognitive training and addressing care needs. The majority of them were free to download, and their primary audience were caregivers, with approximately 40% of them focusing on MCI or dementia patients (Chelberg et al., Reference Chelberg, Neuhaus, Mothershaw, Mahoney and Caffery2021).
Others
In addition to those digital games, serious games were designed and developed for cognitive screening. Users were motivated and engaged to regularly perform screening tasks by playing serious games (Cha et al., Reference Cha, Voigt-Antons, Trahms, O’Sullivan, Gellert, Kuhlmey and Nordheim2019). Karapapas and Goumopoulos (Reference Karapapas and Goumopoulos2021) applied machine learning methods using demographic characteristics and data collected from serious games, which showed a high detection performance. The flexibility of wearable platforms has also provided a variety of data to detect cognitive status (Iaboni et al., Reference Iaboni, Spasojevic, Newman, Schindel, Wang, Ye and Khan2022).
Applications in dementia care and treatment
Socially assistive robots
Given the complexity of dementia care and that the aging population requires more care from a decreasing number of caregivers, researchers have been exploring ways to utilize advanced robotic technology to assist elderly care (Hung et al., Reference Hung, Liu, Woldum, Au-Yeung, Berndt, Wallsworth and Chaudhury2019; Koutentakis et al., Reference Koutentakis, Pilozzi and Huang2020). PARO is one of the most popular interactive pet robots for older adults. It has the appearance of a baby harp seal and provides companionship and emotional interaction to users (Hung et al., Reference Hung, Liu, Woldum, Au-Yeung, Berndt, Wallsworth and Chaudhury2019). Other socially interactive robots provide support in daily engagement for those who have MCI or are at the early stage of dementia (Law et al., Reference Law, Sutherland, Ahn, MacDonald, Peri, Johanson and Broadbent2019). CompanionAble is a robot that helps MCI or dementia patients live at home by linking to a smart home environment (Schroeter et al., Reference Schroeter, Mueller, Volkhardt, Einhorn, Huijnen, van and Gross2013). This robot focuses on cognitive and social support, such as daily activity reminders, suggesting activities, video calling, and cognitive training, which were tested with five couples in their homes over 2 days and potentially reduced the burden for caregivers (Schroeter et al., Reference Schroeter, Mueller, Volkhardt, Einhorn, Huijnen, van and Gross2013). RobuLAB10 is a robot to monitor emotions, help in health emergencies, make calls, and provide cognitive training and other support for daily activities (Pino et al., Reference Pino, Boulay, Jouen and Rigaud2015). These social robots are tested in research with limited participants followed during a short period and are not widely adopted in the real world.
Drug discovery
New drug discovery is a long process, including preclinical processes and clinical trials. The preclinical process before in vitro tests includes target identification and validation, compound screening, and lead discovery (Stamate et al., Reference Stamate, Kim, Proitsi, Westwood, Baird, Nevado-Holgado and Legido-Quigley2019). AI techniques could be applied in all aspects of this process to accelerate new drug development, among which VS and target discovery are the most common application scenarios (Stamate et al., Reference Stamate, Kim, Proitsi, Westwood, Baird, Nevado-Holgado and Legido-Quigley2019). Figure 2 shows the common AI application of dementia drug discovery.

Figure 2. Applications of AI on Dementia Drug Discovery
Target discovery aims to confirm a causal association between target and disease, which involves protein structure and chemical–protein interaction predictions (Fang et al., Reference Fang, Li, Liu, Pang, Li, Yang and Du2015). Recent studies indicated that AD shares intermediate endophenotypes and underlying mechanisms with other diseases, which means there would be multiple targets for AD (Fang et al., Reference Fang, Pieper, Nussinov, Lee, Bekris, Leverenz and Cheng2020). Fang et al. applied naïve Bayesian and recursive partitioning algorithms to predict active compounds bound to as many targets as possible (e.g., the Amyloid Precursor Protein). The methods were evaluated with internally fivefold cross-validation and an external test dataset with an average AUC of 0.965 (Fang et al., Reference Fang, Li, Liu, Pang, Li, Yang and Du2015). His team also proposed a network-based AI framework to identify potential drug targets by integrating multiomics data, human protein–protein networks, and other related data (Fang et al., Reference Fang, Zhang, Wang, Chiang, Zhou, Hou and Cheng2021).
VS is extremely computationally intensive and likely to take an incredible amount of time in silico searches over millions of compounds, ultimately increasing yields of potential drug lead (Carpenter and Huang, Reference Carpenter and Huang2018). Machine learning methods are conducted to speed this process up by building predictive models using active and inactive molecules. SVM is generally among the top performers in machine learning for VS studies (Carpenter and Huang, Reference Carpenter and Huang2018). It applied the “kernel” function to map the database molecules into high-dimensional representations. Yang et al. (Reference Yang, Lv, Chen and Xue2010) performed SVM and RF to predict γ-secretase inhibitors and noninhibitors related to AD prevention and treatment.
Deep learning is reported to perform better than machine learning methods (Carpenter and Huang, Reference Carpenter and Huang2018), among which ANN and CNN are the most widely used methods (Anastasio, Reference Anastasio2021; Reference Fang, Zhang, Wang, Chiang, Zhou, Hou and ChengFang et al., 2022; Wang et al., Reference Wang, Du, Ding, Rodriguez-Paton and Song2022).Rodriguez et al. (Reference Rodriguez, Hug, Todorov, Moret, Boswell, Evans and Sokolov2021) proposed a machine learning framework to nominate drugs that the FDA had already approved. They explored the potential associations between AD and molecular mechanisms described by a list of genes, which integrated LR, SVM, RF, and two-layer CNN. Wang et al. (Reference Wang, Du, Ding, Rodriguez-Paton and Song2022) applied graph-based deep learning for drug–target interaction prediction, which performed better than naïve Bayes, logistic regression, and RF classifiers.
Conclusions and future challenges
AI technology can be applied to the field of dementia research to contribute to fast and accurate diagnosis, providing accessible cognition training tools and reducing care burden. AI can also monitor the progression from MCI to dementia so that those at high risk receive timely interventions. Deep learning has become more popular, particularly in image processing, due to its ability to process complex data. However, one the one hand, the complexity algorithms and black box of explanation restrict its access to clinical researchers. On the other hand, most of the studies explored the performance of the proposed algorithms on datasets with different sample sizes and data features, which makes it challenging to compare different methods. Online datasets, such as ADNI, AIBL, and MIRIAD, have contributed to dementia research (Ebrahimighahnavieh et al., Reference Ebrahimighahnavieh, Luo and Chiong2020). However, even in the same datasets, different studies may still be incomparable as they can apply different parts of those data for different features. For example, applying single biomarkers or merging various data sources, such as demographic and clinical information, performs differently. Thus, further exploration of benchmarking datasets and standard frameworks in different types of dementia is a necessary challenge. In addition, the increasing usage of smart wearable devices that generate complexity big data has provided a new avenue for detecting cognitive impairment (Chen et al., Reference Chen, Jankovic, Marinsek, Foschini, Kourtis, Signorini and Trister2019). This kind of digital biomarker has enormous potential power in dementia research in the future.
Open peer review
To view the open peer review materials for this article, please visit http://doi.org/10.1017/pcm.2022.10.
Acknowledgements
This manuscript was facilitated by the Alzheimer’s Association International Society to Advance Alzheimer’s Research and Treatment (ISTAART), through the Design and Data Analytics professional interest area (DaDA PIA). The views and opinions expressed by the authors in this publication represent those of the authors and do not necessarily reflect those of the PIA membership, ISTAART, or the Alzheimer’s Association.
Author contributions
K.K.F.T. and P.J. authors made an equal contribution to this work.
Competing interest
The authors declare none.






Comments
No accompanying comment.